Tetrachloroterephthalonitrile didn’t burst onto the scene by accident. Back in the years when the chemical industry searched for potent molecules to toughen up plastics and produce dyes, scientists spotted the potential in arranging a benzene ring, sticking four chlorine atoms on it, then swapping in two nitrile groups. Industry experts pushed for robust materials, especially after the Second World War, and chemical researchers leaned hard into organochlorine derivatives. The sharp rise in demand for agrochemicals and pigments drove the markets, so tetrachloroterephthalonitrile caught the eye for its structure and stability. In old patent archives from the 1950s and 60s, records show several global companies chasing new nitrile-based chemicals, hoping they could serve as backbone molecules in emerging technologies. Chemical plants in Europe and the US scaled up production in the late 20th century once farmers started relying on new families of herbicides. Several trade disputes broke out over the purity and labeling of such nitrile chemicals—something that foreshadowed today’s regulatory concerns.
If you pick up a container clearly labeled as tetrachloroterephthalonitrile or punch up its CAS number—117-18-0—the crystalline powder looks almost white or slightly off-white, and sometimes it almost disappears in organic solvents. It gives off little or no odor, doesn’t catch fire easily, sticks stubbornly to most glassware, and clumps together if the humidity runs high. Technical datasheets usually boast about the high melting point up past 200°C, with solubility data included for acetone, chloroform, or a handful of chlorinated solvents. Its density hovers around 1.6 g/cm³. You might see it traded as “TCTN” or “Tetrachloro-1,4-dicyanobenzene” in old industry literature or under product names spun by big chemical suppliers. Standard packaging runs from 25-kg craft drums to sealed LDPE-lined sacks, always with bold hazard marks in black and white.
Spec sheets for this compound don’t mince words. Purity sits above 98% for most market-grade batches. Producers must spell out trace contaminants: leftover chlorinated byproducts, moisture, and sometimes trace metals creep in from reactor corrosion. Proper label warnings jump out: “Harmful if swallowed. Handle with care. Environmental Hazard.” The GHS pictograms on containers flag hazards and instructions focus on closed-system operations, splash-proof goggles, N95 masks, and heavy gloves. MSDS sheets echo these points. Shelf life for properly stored product stretches past two years without much degradation, but containers can’t take sunlight or temperature swings, since photochemical reactions sometimes create stinkier, more dangerous chlorinated byproducts.
Chemists usually take the route of chlorinating terephthalonitrile with an excess of chlorine gas under high temperature and pressure. Commercial reactors churn away at over 150°C, bubbling chlorine through a slurry with a suitable Lewis acid catalyst like iron(III) chloride. Yields depend on reaction time and temperature. Careful controls stop unwanted over-chlorination or creation of disoriented isomers. Sometimes companies lean on batch synthesis, but continuous-feed systems run round the clock for large orders. Workers separate crude solids from the reaction kettle, strip away unreacted starting materials with solvents, then purify the product with multiple recrystallizations. Waste-handling becomes a headache, with spent acid, discarded solvents, and residual chlorinated organics piling up after each run—a big reason regulators demand strict emissions records from plant operators.
Tetrachloroterephthalonitrile’s layout makes it a rock-solid base for chemical changes. The nitrile groups open doors for nucleophilic substitutions, while the chlorines can swing out for arylation reactions or formation of potent herbicidal intermediates. Technicians in specialty labs use it as a launching pad for building complex, ring-fused dyes or specialty polymers. Researchers discovered that heating the compound with potassium hydroxide strips off nitrile groups for new aromatic acids. Pharmaceutical chemists have tinkered with related nitrile compounds, trying to snip and add side groups to build new drug scaffolds, though tetrachloro versions rarely make it past early toxicity screens. Industrial teams still focus on finding ways to tame the structure for colorfast pigments and corrosion-resistant coatings.
You can thumb through old chemical catalogues and find this molecule parading as “TCTN,” “Tetrachlorodicyanobenzene,” or, in some catalogs, “2,3,5,6-Tetrachloroterephthalonitrile.” Several European and Asian manufacturers use code names blended with company initials, which muddies traceability. Safety authorities always call for the official IUPAC name on container labeling, especially for customs and cross-border shipping, to avoid mishaps. These names might bore the average technician, but they prevent accidental mix-ups, especially between similar-looking chlorinated aromatics with wildly different toxicity or regulatory status.
Handling this powder tests the patience of even the most seasoned chemical workers. Skin contact doesn’t burn on the spot but prolonged exposure makes rashes flare up, and inhaling dust causes headaches and sore throats. Spills sometimes linger on plant floors, since the low vapor pressure keeps it from wafting away. Regulations in North America and Europe push for closed-system transfer pipelines, dust extraction, and double bagging during transport. Most modern labs count on regular air monitoring and ask workers to shower and swap clothes at shift’s end. Accidental exposure pushes plant medics to run liver and kidney checks. Older case histories remind us: not every company kept those standards high in past decades, and accidental releases poisoned wildlife in more than one documented spill. Lessons learned shaped today’s tighter safety codes and harsher penalties for corner-cutting.
Few chemicals find such solid footing in so many industries as tetrachloroterephthalonitrile. It’s run in batches for specialty herbicides, where just a nip gets field weeds out of the way before they destroy crops. PCB manufacturers lean on it for etching agents and keeping circuit traces crisp. Paint and pigment companies mix it into colorants to lock in shade durability under harsh weather. Newer applications include blending it into high-performance plastics, especially for structural parts in electronics that have to stand up to heat or chemical spills. R&D chemists dabble with the compound as an intermediate, using it to shape new organic compounds for materials science. Even so, regulators keep a wary eye on end products, noting that the reactivity and persistence of aromatic nitriles can sometimes trip regulatory alarms over environmental persistence.
Chemists in academic labs rarely skip over tetrachloroterephthalonitrile when they dig into the interaction of chlorinated aromatics with nucleophiles or hunt for new ways to toughen synthetic fibers. University research has shown the molecule resists UV breakdown longer than many other chlorinated compounds. Studies in polymer science hunt for ways to snap the chlorine bonds or tailor the aromatic core for niche functions like selective adsorption or advanced sensor materials. Corporate patent filings keep stacking up, especially where new formulations can knock down production costs for agrochemicals or electronics.
No one can dodge the health questions about aromatic nitriles like this one. Animal studies pulled up clear warnings: high-dose exposures thump the kidneys and liver, with some low-level chronic effects reported on hematological parameters. The molecule doesn’t break down easily once it hits the soil; it lingers, stacks up in sediment, and shows up in the tissues of freshwater species near discharge sites. Workers exposed before the 1990s—before dust controls improved—reported breathing trouble, skin dryness, and chemical sensitivity. Health agencies urge manufacturers to capture emissions right at the source, run regular plant hygiene checks, and test local waterways near waste-handling plants. Missed steps can—and have—spelled long-term environmental headaches, so responsible operators stay alert to new research about breakdown pathways and safe disposal.
Scientific curiosity refuses to stand still, so tetrachloroterephthalonitrile continues to spark interest in labs pushing boundaries in organic synthesis, printed electronics, and agrochemical reformulation. Fresh research aims to crack the stubborn environmental persistence by finding catalytic systems that can safely dechlorinate the molecule under mild conditions. Some startups trial biocatalytic treatments using engineered microbes, seeking ways to stack environmental safety against industrial yield. Others look at upcycling spent tetrachloroterephthalonitrile into value-added specialty materials, using the robust aromatic framework to craft new, more biodegradable molecules. Regulatory changes in Europe, typically ahead of other regions, could press for even tougher controls on aromatic chlorinated compounds, possibly driving industry to seek replacements or design cleaner synthetic routes. This interplay of chemistry, health, safety, and innovation guarantees that researchers, regulators, and plant operators can’t take their eyes off tetrachloroterephthalonitrile anytime soon.
At first glance, tetrachloroterephthalonitrile sounds like a word no one wants to pronounce, much less think about. Yet, this chemical has found a spot on factory shelves and in the supply chains of businesses that make things most folks rarely consider. In particular, it’s a key player in the production of specialty pesticides, helping protect crops from the kinds of fungi and pests that might turn a good harvest bad.
The appeal lies in its strength as an active ingredient. Chemicals built from this base get sprayed in agriculture, showing up in fungicides that work hard on fields before anyone lines up at a supermarket. In the world of farming, pests and diseases carve deep holes in profits, so tools that can keep those problems away turn into money-savers for growers.
Factories don’t choose ingredients like tetrachloroterephthalonitrile on a whim. It comes down to performance and cost. Compared with other chemical building blocks, this one brings a mix of power and predictability. Once those downstream companies have their hands on it, they’re not just selling chemical barrels—they’re supporting the entire food supply chain.
Besides agriculture, this chemical occasionally finds its way to specialty coatings. These might land on wires, electronic parts, or even heavy-duty pipe systems. What stands out is the need for protective properties, especially resistance to wear, moisture, and aggressive environments.
Chemistry gives us tools, but every tool cuts both ways. Manufacturing and applying substances like tetrachloroterephthalonitrile means workers and communities get exposed to traces, whether through leaks, spills, or lingering dust. I’ve seen this kind of worry in conversations with folks who live near industrial plants. Stories about odd smells or recurring health problems don’t always get much attention, yet over time, health officials have flagged long-term effects linked to exposure.
Government rules exist because these risks can stack up. Regulatory agencies watch for high build-ups in soil or water, especially since some breakdown products stick around for decades. It used to be easy for companies to write off concerns as “industry business,” but stricter limits on emissions and workplace conditions now ask more from management. Real change happens on the plant floor, where better training, airtight gloves, better air systems, and careful waste handling actually make a difference.
In a job long ago, I spent time touring chemical factories. The plant manager pointed to his stacks of barrels and said, “People need these; just not in their backyard.” Factories can reduce danger with smart containment and honest reporting, but nothing beats double-checking every shipment, every hose connection, and every waste tank.
As for replacements, scientists look hard for options that break down faster and stick to their targets, not the whole environment. In some markets, demand for “greener” solutions is real, driving research shifts and even funding for smaller startups. Even if these new answers cost more up front, the savings show up later—in health, in reclaimed soil, and in giving neighbors peace of mind.
Tetrachloroterephthalonitrile isn’t going away soon. As long as it delivers results in farming and manufacturing, companies will keep using it. Ordinary folks rarely know the name, but the choices made out in the fields and factories shape the food on our plates and the air we breathe. Companies, regulators, and neighbors do better by sitting at the same table, sharing real numbers and new ideas—because ignoring these details only lets yesterday’s mistakes sneak into tomorrow.
Tetrachloroterephthalonitrile sounds like a jumble, but it’s just one of those tough chemicals that pop up in certain industrial processes. Anyone who’s ever worked with raw materials or specialty chemicals knows the drill: a slipup can land you in trouble pretty quick. This compound, with its mouthful of a name, brings strong reactivity and a reputation for causing bad skin and lung reactions. I once heard from a colleague about getting a rash after just a few minutes without gloves — and the discomfort lasted for days. With stakes like that, people forget how a single shortcut can end up sidelining you.
Some folks treat chemical-handling instructions like a formality, tossing on light rubber gloves or thin dust masks. Tetrachloroterephthalonitrile doesn’t mess around, though. Direct skin contact can trigger burns and persistent irritation. That means real chemical-resistant gloves, not the ones you grab to wash dishes. Lab coats should fit right and stay closed, and old jeans just don’t cut it if dust or splashes get airborne.
Eye protection takes priority too. Safety goggles with side shields, not simple glasses. A splash in your eyes can mean an expensive, painful ER visit. Some labs and factories with lots of powder in the air also line up face shields for full protection. I’ve seen people regret skipping this step after dealing with red, irritated skin from constant exposure. It’s tempting to ditch the mask or goggles halfway through a shift, but health doesn’t bank on luck.
Once, in a cramped workspace, poor ventilation turned a small chemical mishap into a real scare. Tetrachloroterephthalonitrile can irritate the nose, throat, and lungs if you breathe in dust or fumes. Extraction fans, local exhaust systems, and simple open windows all help. Workers shouldn’t handle this stuff in closed or stuffy rooms. Wash stations and emergency showers close by are more than a regulatory box-check—they’re essentials, just like fire extinguishers in a kitchen.
Even with proper gear, inhaling fine dust can sneak up on you after repeated exposure. Wearing a tight-seal respirator, not flimsy masks, keeps the risk down. Simple habits like checking the seal and swapping out filters turn into literal lifesavers.
Storing dangerous chemicals just anywhere invites future accidents. Locked metal cabinets, clear labeling, and keeping incompatible substances separated all help avoid messy reactions or fires. I’ve worked in shops where old labels caused confusion — a fast solution is relabeling anything faded or peeling right away.
If a spill hits the bench or floor, nobody should scoop it with bare hands or spread it around with a paper towel. Plenty of workers learn hard lessons covering up mistakes instead of dealing with them head-on. Absorbents made to trap chemical dust get used, along with proper bags for cleanup. Waste stays away from regular trash. Once, someone chucked a contaminated rag in the normal bin, and cleanup after hours took a full team three times longer than it should’ve.
Basic instructions only stick with honest drills and reminders. Periodic training keeps new folks and veterans sharp. Posting cheat sheets and emergency contacts cuts down confusion in stressful moments. It’s easy to get casual after a few months with no problems, but real safety comes from keeping these habits going. I’ve seen experienced crew members share tricks—like checking for leaks with bits of tissue—that wouldn’t appear in a manual. Every little thing adds up in the end.
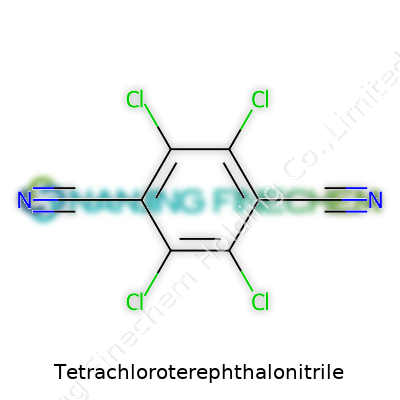
Ask a chemist about Tetrachloroterephthalonitrile, and you’ll see a familiar routine: people hunt for structure in the swirl of consonants and syllables. Chemistry likes to hide meaning in plain sight—this chunky name tells you what’s going on in that molecule. Terephthalonitrile means you get two cyano (–C≡N) groups on a benzene ring, sitting on opposite sides. “Tetrachloro” means the ring is loaded up with four chlorine atoms. Put it together: the chemical formula is C8Cl4N2.
It’s tempting to lump things like Tetrachloroterephthalonitrile in the “industrial stuff only” category. Still, molecules like this turn up in the everyday world in quiet ways. This one sits in the background making life a bit easier for folks in the polymer and chemical manufacturing business. Stuff like flame retardants, specialty coatings, and even certain pesticides often need chemical building blocks that stay stable and hold up under tough conditions. Molecules with hardy chlorine atoms do the trick.
Let’s face it—safety concerns about chlorinated chemicals crop up for a reason. Chlorine doesn’t let go easily. It isn’t just a feature that helps keep plastics from catching fire. If you’re not careful about how you release or break these molecules, you’re left with a mess for the environment, and sometimes your health. Some chlorinated compounds have a notorious history—think dioxins. Others might not break down quickly, building up in soils or water supplies.
I remember working in a university lab that dealt with aromatic nitriles. You grow an appreciation for precise labeling, proper storage, and never rushing waste disposal. The temptation in any busy chemical process is to focus on the immediate product and forget about tomorrow’s trash. Tetrachloroterephthalonitrile doesn’t ask for much daily attention, but let it escape, and you have a headache no PPE gear really solves. In industry, chemists and engineers constantly navigate how to balance the world’s hunger for durable, affordable materials against risks stirred up along the way.
There’s no switch that instantly flips complicated chemistry into something “green.” The formula C8Cl4N2 captures a lot of stability, but also raises flags if mismanaged. To dial down trouble, the solution starts with knowledge. Research teams keep scanning for ways to handle, recycle, or outright swap out tricky compounds. Some startup labs focus on catalysts that let you make the same kinds of materials with less chlorine. Others develop methods for trapping or transforming hazardous byproducts even before they make it out of the stack or drain.
At a personal level, whether you whip up a reaction at the bench or manage a process plant, buy-in starts with a willingness to understand the stuff being handled. Reading an MSDS sheet is less a legal chore and more a way to map out what could go wrong and close a few doors before anyone walks through them unprepared. Recognizing the value and risks in every big, gnarly chemical formula is how the industry moves forward without tripping over its own tail.
Maybe most folks never need to recite “C8Cl4N2.” Still, lives don’t move forward without raw materials, and it’s easy to take them for granted until something goes sideways. Putting effort into understanding—and respecting—molecules like Tetrachloroterephthalonitrile is what lets the smartest solutions rise to the top, in the lab and far beyond it.
Storing chemicals with long, winding names like tetrachloroterephthalonitrile can seem intimidating, but the approach demands respect, not confusion. As someone who’s clocked plenty of hours in both research labs and basic storage rooms, I can say: underestimating a compound like this often leads to headaches or worse. Safety isn’t just paperwork—it's the difference between routine work and a very bad news story.
Finding a spot isn’t about tossing a drum on the nearest shelf. Strong shelves, made of steel or sturdy plastic, make a solid foundation. This stuff doesn’t react kindly to random moisture or sunlight. Sun through a warehouse skylight heats containers and starts chemical changes nobody wants. Darkness makes life easier for everyone. Secure shelving also means nothing slides off in an earthquake or gives way when someone bumps into it with a dolly.
Forget the urge to transfer this material into old soda bottles or leftover paint cans. Factory-sealed, chemical-resistant containers block leaks and slow down any nasty reactions with the outside world. Lid fits tight—no slipperiness around the edges. If a container starts showing cracks, get rid of it the right way, not into the main trash. Use heavy-duty labels for every drum, no lazy scribbles. If someone’s cleaning the warehouse after a long shift, they still know what they’re handling before lifting.
Cracking a window won't do the trick with airborne hazards. A proper ventilation system pulls any vapors out and away, dropping risk for everyone. Some facilities use exhaust fans or negative pressure setups in their chemical storage rooms, and they see fewer incidents. A basic fan in the corner only stirs dust. Good airflow with reliable equipment shields workers and neighbors.
Dampness turns ordinary storage into hazardous storage. Tetrachloroterephthalonitrile in contact with humidity or water can go from stable to troublesome. Dry rooms with a low relative humidity prove their value again and again. Some places combine climate controls and sealed storage to keep everything bone dry. Desiccant packs sometimes help, but machinery that regulates humidity brings real peace of mind during rainy months or in coastal areas.
Jamming all chemicals into one closet doesn’t just make for a crowded shelf. Separate out incompatible chemicals. I’ve seen warehouses with color-coded floor tape marking off pesticide sections away from acids or oxidizers. Grouping by chemical family or hazard reduces the risk of accidental mixing and allows for faster first response if something spills.
Sophisticated rules don’t help if staff overlooks them. Routine walks through the storage area, checking for spills, weak containers, or rogue labels catch problems before they grow. Annual training means everyone, even veterans, stays sharp on procedures, emergency cleanups, and knowing which gloves or masks to grab. Sharing real stories about near-misses means new hires pay attention, not just read a checklist.
Fires, leaks, and spills turn safe storage into chaos in seconds. Emergency eye-wash stations and showers sit nearby for a reason. Fire extinguishers—right type, fully charged—shouldn’t gather dust. Familiarity with local hazmat numbers and clear, posted evacuation routes give everyone a path out if an accident unfolds. Nobody expects trouble. That’s why drills keep surprises to a minimum.
Smart storage for chemicals like tetrachloroterephthalonitrile isn’t flashy. It’s all about small, daily choices. Dry rooms, snug lids, tough shelves, tested emergency plans, and real teamwork build a safer workplace. If we give the compound the attention it wants, it never gets a chance to remind us why we should have listened.
Tetrachloroterephthalonitrile, often abbreviated as TCTPN, pops up mostly in the production of certain resins, dyes, and other specialty chemicals. In industrial settings, this is not a substance people encounter by accident. It tends to stay in factories and labs, stored in heavy drums with big warning stickers. Still, what starts in a factory can have a way of finding its way out, whether through leaks, improper waste handling, or in the unlucky case of an accident.
Contact with TCTPN isn’t something to take lightly. Chemical manufacturers and workers have to worry about skin and eye irritation, among other direct symptoms. Some research points to respiratory issues from long-term exposure. It’s pretty harsh: folks I’ve spoken with who’ve worked near it always describe wearing such thick gear they can barely move. That’s not just because of company policy — it’s because one drop or a whiff on an unprotected patch of skin and you’re off to the clinic.
Medical studies focus on acute reactions like rashes, breathing problems, and headaches. EPA profiles show potential for longer-term problems, including organ toxicity, if someone is around higher concentrations for too long. The trouble is, many chemicals in its class haven’t been around in large amounts for decades. So, we get patchy data, a lot of caution, and big gaps in long-term studies. Most people will never get exposed, but the risk for those who do is very real.
The environmental impact spells further trouble. TCTPN sticks around. Tests show that it doesn’t break down quickly in soil or water. I’ve seen environmental reports from plants that point to serious contamination issues: once it gets into rivers or groundwater, removing it costs big money and takes years. Fish and wildlife that live downstream suffer. Their habitats get poisoned, leading sometimes to die-offs that ripple through local ecosystems.
This stuff also travels. Wind can blow powder away, and spills seep through the ground far from the original site. Scientists in environmental chemistry warn that small leaks, if ignored, can add up to real problems for nearby communities relying on wells or local crops that pick up toxins from soil.
Mitigation comes down to prevention. Industrial facilities can’t just dump waste outside and hope it disappears. Strict rules matter. Over the years, I’ve seen state regulators tighten inspections and demand safer storage, but enforcement varies. Some countries set high standards, others look the other way or lack funding for spot checks. In my experience talking with plant managers, employee training ends up just as critical as fancy equipment. People make mistakes when cutting corners.
Better technology helps. Closed-loop systems and leak detection sensors, once rare, help catch problems early. Labs now run more regular checks on soil and water near chemical plants. Companies caught polluting ought to face steep penalties, enough to make it cheaper to play by the rules than risk a fine.
It helps to let communities in on what’s happening. If people living near factories know what chemicals move in and out, they’ll speak up quicker about problems. Public pressure has led to cleaner practices at some of the dirtiest industrial sites I’ve visited. It doesn’t solve everything, but it does keep everyone a bit more honest.
In the end, while TCTPN enables the production of various modern materials, it carries enough risk to warrant real caution. Factories must invest in both technology and people, keep waste contained, and fix leaks before minor problems spill out into major disasters. Regulators and locals can’t afford to let their guard down — cleanups never come cheap, and health is tough to recover once lost.
| Names | |
| Preferred IUPAC name | 1,2,4,5-tetrachlorobenzene-1,4-dicarbonitrile |
| Other names |
Chlorothalonil Bravo Daconil Tetrachloroisophthalonitrile TCIN Nipacide |
| Pronunciation | /ˌtɛtrəˌklɔːroʊˌtɛrəfθæˈlɒnɪtraɪl/ |
| Identifiers | |
| CAS Number | 117-18-0 |
| Beilstein Reference | 1221189 |
| ChEBI | CHEBI:34915 |
| ChEMBL | CHEMBL2106429 |
| ChemSpider | 20892501 |
| DrugBank | DB08746 |
| ECHA InfoCard | 100.014.237 |
| EC Number | 201-895-1 |
| Gmelin Reference | 456384 |
| KEGG | C18668 |
| MeSH | D013742 |
| PubChem CID | 85700 |
| RTECS number | WZ1925000 |
| UNII | LD9H03R60H |
| UN number | UN3347 |
| CompTox Dashboard (EPA) | `DTXSID7020667` |
| Properties | |
| Chemical formula | C8Cl4N2 |
| Molar mass | 290.93 g/mol |
| Appearance | White to light tan crystalline solid |
| Odor | Odorless |
| Density | 1.74 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 2.37E-7 mmHg at 25°C |
| Acidity (pKa) | 1.09 |
| Magnetic susceptibility (χ) | -0.0000936 |
| Refractive index (nD) | 1.6400 |
| Viscosity | 1.82e-2 Pa·s (25 °C) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 369.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -117.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -864 kJ/mol |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes skin irritation; causes serious eye irritation; may cause respiratory irritation; very toxic to aquatic life. |
| GHS labelling | GHS02, GHS07, GHS08, GHS09 |
| Pictograms | GHS06 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P284, P302+P352, P304+P340, P308+P311, P312, P322, P333+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0-㋑ |
| Flash point | > 251°C (closed cup) |
| Autoignition temperature | 730°C |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 oral rat 4000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 8900 mg/kg |
| NIOSH | SN1575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | 20 mg/m3 |
| Related compounds | |
| Related compounds |
Phosgene Terephthalonitrile Chloranil |