The story of N-Butylamine traces back to a period when chemists pursued new organic compounds by experimenting with the basics—alkylation and amination of straight-chain hydrocarbons. Early documentation shows labs in Europe around the late 19th century isolating aliphatic amines through distillation and reaction with ammonia, often in rough, glassware-filled workrooms where safety meant little more than an open window. The pioneers didn’t have fume hoods, and their notes reveal burnt skin, headaches, and more than one explosion from trial-and-error runs. With time, synthesis methods grew more refined, and commercial interest picked up once uses in pharmaceuticals, pesticides, and rubber production came into focus. Bringing N-Butylamine beyond bench-scale birthed new standards for purity and worker protection, with companies recognizing both the hazards and profits tied to this manageable, yet volatile amine.
In my work, N-Butylamine stands out as one of those versatile chemicals that seems to slip between different industries with ease. Manufacturers bottle it up mainly in drums or bulk containers, shipping it as a colorless, caustic liquid that smells like old fish bins—the ammonia tang coats your nostrils. Tech datasheets spell out a simple ingredient list, but there’s a world hiding under those purity numbers. Each supplier touts their own batch’s water content, hopes for under 0.5%, and field complaints pour in once traces of corrosion appear on metal pipes or container seams. Chemists clamor for guarantees that residues from previous production won’t alter reactions downstream, and procurement officers keep a wary eye on costs, as raw material prices bounce with the petrochemical markets.
Over the years, I’ve handled N-Butylamine in labs and plant environments, and one thing becomes clear: its volatility is no joke. The boiling point sits uncomfortably low at around 77°C, which means any open flask or valve leak sends clouds of vapor swirling. Vapor forms a flammable mix with air, and a spark turns the workplace into a disaster scene. The density, sitting close to 0.74 g/cm³ at room temperature, makes spills race across floors, seeking cracks and drains. Miscibility with water turns small drips into diluted but persistent odors; nothing hides from the sharp sting on your skin or eyes. The base strength shows up as you watch glass or rubber stoppers etched away, making clear the need for compatible gaskets and seals in every application.
Regulators in every region specify grades and put up barriers on how N-Butylamine must move from point A to B. The labels shout danger: UN 1125, flammable liquid, toxic by inhalation. It’s easy to spot mistakes in old labs—outdated hazard squares, labels faded so workers reach for a bottle assuming water, only to burn fingers. Technical specifications break down so precisely in some markets—99.5% minimum purity, water content, weight per liter, trace metals kept below parts per billion. These norms aren’t just ambitions; letting specs slip leads to failed batches and ruined reactors.
Through personal experience, one of the first prep methods involved the reaction of 1-chlorobutane with ammonia, a classical nucleophilic substitution route. This process runs best in sealed systems under pressure to capture all the amine, as open setups lose too much to the air. Some plants swap out the chlorine for alcohols, running butanol with ammonia in the presence of a catalyst—alumina mesh or zeolites. These tweaks depend on costs and environmental taxes on halide waste. Engineers optimize the sequence to prevent overheating, watching temperature gauges closely since, above a certain threshold, N-Butylamine decomposes or spews out in bursts that nobody wants inside a closed loop.
On my shelf, I keep samples that show just how reactive this amine becomes in the right context. React it with carboxylic acids and you get amides, so pharmaceutical chemists rely on it. Add alkyl halides and you cycle towards secondary, then tertiary amines. N-Butylamine shakes hands with epoxides, isocyanates, and blends into the backbone of dyes and fungicides, picking up alterations as needed. Try it in reductive amination and the flexibility starts showing—building blocks for complex molecules appear, each one tailored in yield or selectivity by choosing this humble C4 amine over a shorter or longer cousin. Its basicity also lets it serve as a scavenger for acidic gases, a trick used in some scrubber systems to clean industrial exhaust streams.
People working across continents call N-Butylamine by many names: 1-Butanamine in formal IUPAC; Butylamine or n-Butylamine by most traders; outside, some refer to C4H9NH2 as Butan-1-amine in research journals. Catalogs list CAS number 109-73-9, so supply chains focus on digits to avoid mislabeling. Each alias pops up in purchasing, R&D, and shipping paperwork. In the end, they all point to the clear, biting liquid with the four-carbon backbone and primary amine.
Nobody forgets their first encounter with a splash of N-Butylamine. Gloves melt if chosen poorly; exposed skin burns and itches for hours. The low flash point—in the low teens Celsius—forces strict bans on open flames. I’ve watched teams plug ventilation gaps by rigging makeshift fans, only to recognize proper fume extraction really can’t be faked. Training sessions push respiratory masks, splash goggles, chemical suits, and quick access to eyewash stations. Storage requires steel drums or tanks lined with corrosion-resistant coatings. Containers need perpetual grounding straps, and everyone respects the rule: Never open in confined spaces. Only through drills and adherence to these hard-learned routines does a lab or factory keep its accident log empty.
Walk into any specialty chemical plant or agrochemical facility, and N-Butylamine has probably passed through at some stage. It ends up as an intermediate in synthesizing pharmaceuticals—a key amide or a starting amine loop. Manufacturers blend it into fungicides and herbicides, driving yields and reducing spray costs in commercial farms. Rubber producers use it in accelerators, shaping tires and hoses that end up on cars and industrial rigs worldwide. Textile processors count on it to build dyes, and oilfield engineers use specialty N-Butylamine derivatives as corrosion inhibitors or anti-scaling blends. Adhesive factories call for it to boost polymer chain growth, chasing better sticking power with each tweak.
R&D teams rarely stand still with commodity chemicals like N-Butylamine. Scientists tinker with catalytic systems aiming to bump yields, searching for greener routes that skip halides or lower pressure requirements. In drug discovery, its amide-making skills spark scaffold innovation, helping labs rush out candidates for tests against infections or cancer. In the energy sector, researchers are considering N-Butylamine as a carrier, linking carbon capture to gas-separating membranes. Digital modeling now predicts reaction pathways, and researchers probe for selectivity by mapping out hydrogen-bonding arrangements on computer clusters.
Toxicologists learn quickly that N-Butylamine doesn’t forgive mistakes. Exposure data from rodents shows a mix of respiratory irritation, central nervous depression, and long-term organ impact if dosing continues. The compound carries a risk of skin sensitization; one drop on a gloved hand, and an itch crawls under the surface. Large-scale exposure studies in industrial settings uncovered a pattern: workers subjected to poor ventilation reported headaches, nausea, and in some rare cases, respiratory distress serious enough to require hospitalization. Regulators responded by testing air quality and enforcing short- and long-term exposure limits. Researchers in universities press on to understand the metabolic breakdown in living beings, tracking byproducts as they circulate through tissues and organs, preparing for the day when new data calls for stricter rules.
Looking ahead, the role of N-Butylamine seems tied to how industries adapt to sustainability and new regulatory landscapes. Green chemistry advocates search for next-gen synthesis methods, rooting out petroleum dependence and lowering energy needs across the chemical sector. Markets in Asia and Latin America are ramping demand for precursors, chasing competitive advantages in agrochemicals and industrial polymers. Environmental agencies, watching the growing traffic, ramp inspections and update transport standards. Research labs could turn out engineered amines tuned for medicinal chemistry or smart materials, where basic building blocks get customized through digital design before a flask opens. As industries recalibrate, those who handle N-Butylamine day-to-day know their vigilance and adaptability matter most for keeping everyone safe and the market steady.
Think of N-Butylamine as one of those quiet contributors hiding in the background of modern chemistry. Sure, most folks won’t find a bottle of it under the kitchen sink, but trace its odd-smelling trail and you’ll wind up in farming, medicine, and even the blue jeans hanging in the closet. On the surface, it looks like a plain, colorless liquid, but its use stretches far beyond what might fit into a basic safety manual.
Agriculture has leaned on N-Butylamine for decades. It doesn’t show up in the fields by itself, but as part of herbicide formulas, it strengthens the fight against weeds that threaten crops. The stuff helps farmers grow cleaner fields and get decent yields. Those of us who rarely see weeds in our boxed lettuce owe something to this chemical’s behind-the-scenes work.
One thing that stands out about N-Butylamine is its role as a building block. Chemists use it to manufacture dyes, rubber chemicals, and pharmaceutical products. Dive into the supply chain for blue jeans or athletic shoes, and you’ll find it working in the process of creating dyes and synthetic rubber. In practice, that translates to more colorfast clothes and reliable tires. While folks rarely connect their daily essentials with a molecule like N-Butylamine, its fingerprint is all over these goods.
There’s also a medical angle. Pharmaceutical companies use compounds that stem from N-Butylamine for drugs that treat infections and other diseases. The presence of this ingredient doesn’t jump off prescription labels, but it stands as a quiet link in the chain that brings effective medication to storefronts and hospitals. A lot of modern medicine depends on such connections—links that never make the news even though they keep things humming.
Working with N-Butylamine isn’t for the careless. The odor alone—similar to dead fish—sets off alarms for anyone with experience in a lab. Skin and eye irritation can happen quickly. Take a deep breath in the wrong place, and the vapor burns all the way down. While accidents feel far removed from everyday life, people who process and transport this chemical have to stay alert. Strict safety procedures keep it from spilling into water or soaking into clothing. It pays to remember that what keeps farms productive shouldn’t be drifting into nearby communities or rivers.
A smarter approach looks like updated protective equipment, frequent training, and better leak-detection systems. Choosing containers designed for harsh chemicals limits the risk of breakage or leakage during transport. The trend toward greener chemistry also nudges manufacturers to consider alternatives that deliver the same benefits with fewer risks, although changing old habits in industry can take time. Until new solutions come along, diligence from everyone in the supply chain makes a difference. Mistakes in handling can undo a lot of good, and with N-Butylamine in so many products and processes, mistakes ripple outward more than people think.
Most people never have to worry about what goes into their jeans, tires, or prescription drugs. But for folks in science, industry, or agriculture, N-Butylamine is a familiar companion. Following solid safety habits and staying aware of the chemical’s nature isn’t just good practice—it’s a responsibility.
Most households stash bottles of cleaning products, solvents, or remnants from a home renovation. Tucked somewhere in many supply chains, N-Butylamine is one of those basic chemicals keeping things running, yet almost nobody outside a lab thinks about it. Its chemical formula is C4H11N, but there’s a whole structure hiding within that formula. Each symbol in it has a story and a real-world impact.
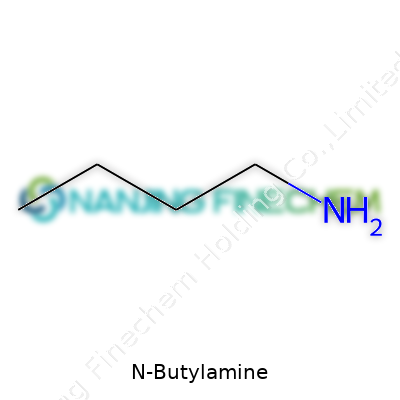
To break it down: C4H11N stands for four carbon atoms, eleven hydrogens, and a single nitrogen atom. This isn’t abstract math; it shows us the backbone and side chain that shape N-Butylamine’s personality. Four carbons line up in a row, one after the next—straight and unbranched, this is what chemists call the “n-butyl” chain. At the very end, a nitrogen sticks out. That one piece turns the chain from an ordinary hydrocarbon into an amine.
Structural formulas matter, especially for anyone who spends time mixing paints, formulating adhesives, or tinkering with chemical processes. N-Butylamine’s arrangement looks like this: CH3-CH2-CH2-CH2-NH2. That NH2 group hangs onto the end of a straight, four-carbon chain—no twists, no side branches. This is what sets “n-butylamine” apart from its isomers like sec-butylamine or tert-butylamine.
Students usually worry about remembering atomic lineups for exams, but the arrangement of atoms really changes everything. The straightforward backbone in N-Butylamine leads to specific boiling points, a sharp, fishy smell, and a behavior in water that makes it popular in the world of agrochemicals, pharmaceuticals, and dyes. These are all tied to the way molecules interact when lined up just right.
Despite only showing up in labs or industrial settings, using N-Butylamine requires careful handling. It evaporates quickly and can burn. In my own experience working near chemical storage, any hint of amine in the air meant the ventilation fans kicked on fast. Just a little exposure gave me a clear headache. The importance of structure doesn’t end with chemical formulas—the way these chemicals interact with the body, the air, or other products all traces back to this molecular map.
Ask a safety officer what’s in a barrel, and recognizing names like N-Butylamine can mean the difference between a regular shift and a dangerous incident. Knowing that the straight chain and terminal amine group mean higher volatility and reactivity isn’t a classroom detail, it’s a lesson learned by anyone who’s ever managed a spill or read a safety datasheet in real life.
Chemical literacy in daily life makes workplaces safer, products more reliable, and troubleshooting much less stressful. Memorizing structures like C4H11N isn’t just for passing exams but for keeping systems running smoothly. Better labeling, accessible safety charts, and routine education about the makeup of basic chemicals really help everyone—not just chemists or engineers—navigate our increasingly complex daily environments.
N-Butylamine shows up in a lot more places than most people notice. Plenty of chemical plants use it as a building block for things like pharmaceuticals, pesticides, and rubber accelerators. It smells kind of fishy, reacts quickly with air and water, and you sometimes see it stored behind warning labels that make people nervous.
Let’s talk health. Just a whiff of N-Butylamine makes your eyes and nose sting, especially if somebody spills it in a lab or factory. Even low concentrations of vapor drive people out of the room coughing. If it gets on your skin, irritation shows up fast. Swallowing a mouthful by accident brings stomach pain, nausea, and a visit to the ER—no home remedies here.
People working with this stuff face bigger risks after months or years. Studies have noted headaches and respiratory troubles in folks who don’t wear decent masks or gloves. There’s little proof N-Butylamine gives you cancer, but the irritation alone disrupts normal work and eats into comfort. People shouldn’t chalk that up as “just part of the job.”
Pouring N-Butylamine down a drain spells trouble for rivers and fish. It doesn’t stick around forever, but fish exposed to it show distress, and even plants in the area wilt from too strong a dose. Overflow or run-off from sloppy factories can move the problem to neighborhoods in just a few rainstorms.
Many local rules and international regulations ban dumping N-Butylamine without proper treatment. This gives neighbors and water systems a fighting chance, but enforcement can get weak if nobody pushes back on cut corners.
In one chemical plant I visited in my late twenties, a tank line split at a weak weld. Staff had just removed their gloves to take lunch, and sharp fumes filled the air within seconds. That day, five people went home early with burns on their arms and throats. Everyone learned firsthand why safety rules aren’t just red tape.
One farmer I met received fertilizer laced with leftover N-Butylamine. That season ended up hurting his tomatoes, and his young nephew developed a nasty cough. Any shortcut in waste disposal makes a real mark on families and their crop yields.
Workplaces that treat N-Butylamine with suspicion avoid trouble. Good ventilation, chemical hoods, and sturdy gloves stand out as necessities, not luxuries. Cleaning up every drop, checking PPE before each shift, and running routine air monitoring saves everyone pain—there’s no honor in acting tough and breathing dangerous fumes.
Communities near plants should keep a nose out for odd smells and watch for unhealthy changes in their yards or streams. Sharing information means people see patterns earlier and don’t brush off mild symptoms until they turn serious.
Disasters and illnesses from N-Butylamine come mostly from ignoring the basics. A few extra minutes double-checking seals and disposal go a long way. In my experience, most workers care deeply about their safety and their families. Everyone in the chain, from management to field worker, does better with straight talk and respect for the risks.
N-Butylamine has a strong smell that fills your nose right away, and it’s well known for being both flammable and corrosive. Plenty of folks out there might only glance at a datasheet, but anyone who's had to mop up a small leak or discover sticky residue on their gloves learns fast that this chemical can cause real trouble if left unchecked. If you're running a shop, teaching chemistry, or simply maintaining a plant, respecting those risks isn’t optional. It’s a routine, not an afterthought.
N-Butylamine doesn’t like heat or sparks, and vapors escape from open containers with little warning. I once worked near a storage room that had aluminum shelves and was poorly ventilated. Someone—maybe new or maybe in a rush—left a bottle cap loose. Hours later, that sharp fishy smell drifted down the hallway, and nobody could stay nearby. It’s clear: air-tight bottles with strong seals do more than keep your workplace tidy. They help prevent headaches, nosebleeds, and in the worst cases, fires.
Nothing beats a good steel safety can with proper labels. Shelving should be chemical-resistant and easy to wipe down, not just whatever’s cheapest. Flammable storage cabinets make all the difference. I’ve spent plenty of afternoons sorting chemicals, and the best labs keep their amines in fire-resistant lockers, well away from oxidizers or acids. Rules posted on walls don’t mean much if the storage looks like an afterthought.
N-Butylamine stings skin and eyes within seconds. A splash can burn, and a lingering vapor irritates lungs. Thick nitrile gloves lay between you and trouble—forget latex, they break down too easily with this compound. Eye protection is standard. After too many stories about folks rubbing their eyes and regretting it, safety glasses stay on my face until the work’s done and hands are washed.
Anyone in a cramped lab without a hood feels the difference in air quality right away. Using this stuff in a fume hood or a breezy, open area cuts down the headaches and dizziness that follow sloppy handling. And if a spill happens, don’t grab paper towels. Spill kits designed for solvents and bases soak up the mess without spreading fumes around.
Storing N-Butylamine near sparks or heat sources is asking for trouble. I’ve watched shops run extension cords over flammable storage just to plug in a kettle—a shortcut that nearly turned into an insurance claim. It doesn’t take much imagination to see where things can go wrong. Keep the stuff far from heat, open flame, or even sunlight streaming through a window onto a shelf. Fire extinguishers rated for chemical and electrical fires should always be nearby, not in a locked closet three doors down.
Treating N-Butylamine seriously every single day counts more than a single annual safety seminar. Label everything. Walk new team members through your procedures in person. Replace cracked stoppers or broken labels right away. If supply costs threaten to eat into budgets, remember: a single spill or injury costs much more than a box of gloves or a new storage cabinet. Industry guidelines push for strict handling, but the real difference comes from daily vigilance by everyone in the room.
Not many people toss the term N-Butylamine into everyday conversation. Still, anybody who’s set foot in a chemistry lab or worked in a plant knows it shows up on ingredient lists. It’s a colorless liquid, smells like fish, and—let’s be honest—isn’t exactly friendly stuff if you ignore the warning labels.
Plenty of folks assume tossing on a pair of gloves counts as safety. While N-Butylamine sounds harmless thanks to the “amine,” this chemical brings some real hazards. Breathing the vapors can leave you dizzy or short of breath. Spill a bit on your hand, and you’ll know quickly that skin takes the hit. A splash in your eyes? That’s not a situation you walk off. On top of that, it’s flammable—those vapors hang around waiting for a spark. Disregard the safety basics and you end up with stories you’d rather forget, if you even get the chance to tell them.
Going through formal chemical safety training shook me out of “it can’t be that bad.” Step into any decent lab or chemical plant, and you’ll see practical safety habits at work. Start with the basics: wear splash-proof goggles. Not the cheap ones that fog up, but proper, tight-fitting goggles. Gloves matter too—nitrile or butyl rubber hold up better than latex with this kind of chemical.
What about the air you’re breathing? N-Butylamine gives off fumes that can choke you up. Good ventilation isn't optional. Fume hoods come in handy for a reason—working outside one, you breathe in more than you’d like. If the job takes you outside the lab onto a loading dock or plant floor, portable fume extractors help. Never mess with a gas mask from the hardware store for these fumes. Only proper respirators rated for organic vapors have your back.
Clothing matters too. Skip those synthetic shirts. N-Butylamine can seep through, turning a minor splash into a problem. Go with full-length lab coats or coveralls. If your work involves pouring or handling drums, face shields add another layer—eyes are too precious to risk.
I've seen what happens when someone flies through clean-up and forgets that N-Butylamine’s vapors catch fire at room temperature. Label your containers. Lock them up when you’re done. Keep all sources of ignition, even a stray spark from static, away from open containers. Fire extinguishers marked for flammable liquids stay within reach for good reason.
Sure, nobody wants to think about emergencies, but planning beats scrambling. Safety showers and eye wash stations shouldn’t sit gathering dust. It sounds excessive until someone takes a hit. Spill kits—ones made for caustic and flammable liquids—belong nearby. Never reach straight for water to clean a large N-Butylamine spill; you want a proper neutralizing solution and absorbents designed for this purpose. Know the evacuation routes. Practice them. It might feel silly during a drill, but muscle memory kicks in fast during a real deal.
Skipping steps, eyeballing measurements, or using worn-out safety gear might shave a few minutes—but it isn't worth your health. Every person I know who's worked around N-Butylamine and walked away unharmed gives credit to stubborn routines, not luck. Gear up, think ahead, and remind the new folks. At the end of the day, everyone wants to walk out with the same number of fingers, toes, and working senses they had in the morning.
| Names | |
| Preferred IUPAC name | butan-1-amine |
| Other names |
1-Butanamine Butylamine n-Butanamine Butamine |
| Pronunciation | /ˌɛnˈbjuːtɪl.əˌmiːn/ |
| Identifiers | |
| CAS Number | 109-73-9 |
| Beilstein Reference | 604068 |
| ChEBI | CHEBI:28997 |
| ChEMBL | CHEMBL14419 |
| ChemSpider | 5931 |
| DrugBank | DB01942 |
| ECHA InfoCard | 03f198b2-e1a5-4c1b-8fd6-20b5992ab14d |
| EC Number | 200-699-2 |
| Gmelin Reference | 62113 |
| KEGG | C01717 |
| MeSH | D020021 |
| PubChem CID | 8113 |
| RTECS number | EO1400000 |
| UNII | 0CK95POG8E |
| UN number | UN1125 |
| Properties | |
| Chemical formula | C4H11N |
| Molar mass | 73.14 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Ammonia-like |
| Density | 0.74 g/cm³ |
| Solubility in water | miscible |
| log P | 0.97 |
| Vapor pressure | 11.9 kPa (at 20 °C) |
| Acidity (pKa) | 10.78 |
| Basicity (pKb) | 3.38 |
| Magnetic susceptibility (χ) | -7.6×10⁻⁹ |
| Refractive index (nD) | 1.397 |
| Viscosity | 0.49 mPa·s (20 °C) |
| Dipole moment | 4.66 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 228.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -74.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3892.3 kJ/mol |
| Pharmacology | |
| ATC code | C01DX17 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02, GHS07, GHS08 |
| Signal word | Danger |
| Hazard statements | H225, H302, H314, H332 |
| Precautionary statements | P210, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P311, P312, P405, P501 |
| NFPA 704 (fire diamond) | 3-3-0 |
| Flash point | -15 °C |
| Autoignition temperature | 290 °C (554 °F) |
| Explosive limits | 1.7% - 10.0% |
| Lethal dose or concentration | LD50 oral rat 366 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat 366 mg/kg |
| NIOSH | NIOSH: SE6650000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of N-Butylamine is "5 ppm (15 mg/m³) |
| REL (Recommended) | 100 ppm |
| IDLH (Immediate danger) | 660 ppm |
| Related compounds | |
| Related compounds |
Methylamine Ethylamine Propylamine Isobutylamine Sec-butylamine tert-Butylamine Aniline |