Walking through the history of aromatic nitrile chemistry brings up steady progress over the decades. Chemists started exploring benzonitriles for their ability to stack new functions onto simple benzene rings, and once the value of trifluoromethyl groups entered the game, research picked up. The drive for pharmaceutically active compounds and new materials kept 4-Methoxy-3-Trifluoromethylbenzonitrile from falling into obscurity. As advanced synthesis methods became routine in labs, manufacturers managed to step up both scale and refinement, giving researchers tools once out of reach. It’s not just a tale of curiosity—demand for complex molecules in crop protection and drug discovery keeps these substituted benzonitriles relevant.
4-Methoxy-3-Trifluoromethylbenzonitrile stands out among benzonitriles because it blends the electron-donating effects of a methoxy group and the electron-withdrawing punch from its trifluoromethyl group. The compound falls into the category of specialty organics: not an everyday item, but when you spot it in a catalog, you know someone nearby dreams up new life-saving compounds or tweaks crop chemistry. Whether you look in specialty chemical catalogs or long lists in research papers, you spot it under a handful of synonyms and product codes, often sourced at high purity for critical experimentation.
4-Methoxy-3-Trifluoromethylbenzonitrile usually appears as a pale, sometimes off-white solid, slipping easily into most lab solvents. The molecule weighs in at 201.15 g/mol, with a melting point typically floating between 55 and 60°C. These trifluoromethyl and methoxy arms dance on the carbon ring, making it relatively resistant to harsh conditions while remaining reactive at just the right spots. It sports a notable resistance to hydrolysis, thanks to the nitrile group’s tight structure, but keeps a manageable boiling point for small-lab distillations. In my hands, I’ve seen how it clings to silica during chromatography, probably because of the combination of polar and lipophilic fragments.
Analyzing a bottle’s label reveals key details: around 98% purity or above, minor moisture warning (since water stubbornly clings during storage), and regulated storage protocols—think somewhere cool, away from reactive acids or bases. Suppliers usually throw in infra-red (IR), NMR, and mass spectra for batch authentication, so that no one ends up with unexpected by-products or isomers. Lot numbers stay critical for batch tracking. Standard labeling covers its synonyms, hazard warnings, and CAS number (149793-69-1), vital for anyone hunting it across supply chains.
Getting your hands dirty with benzonitrile synthesis means picking a strategy for both the trifluoromethyl and methoxy groups. Most labs prefer starting from a protected phenol derivative, using classic Sandmeyer reactions to plant the nitrile function. Others trek through methylation of a hydroxybenzonitrile, then slap on the trifluoromethyl group with copper or nickel catalysis. If you care about yield and byproduct control, copper-catalyzed trifluoromethylation is usually the weapon of choice. These reaction setups demand technical skill to prevent side-product headaches; a slipup floods your flask with intractable isomers or polymeric tar.
The real fun starts once you have this benzonitrile. The trifluoromethyl and methoxy groups, sitting across the ring from the nitrile, don’t just mark their territory—they tweak the reactivity for coupling, reduction, and functionalization. Suzuki and Buchwald reactions run reliably, as long as the conditions match the electronics of the ring. Nitrile reduction heads in many directions, from forming the desired amine (a trick I’ve seen done with Raney nickel under mild hydrogenation), to hitting the aldehyde stage if you keep your patience and control. Methoxy deprotection converts the compound into a phenol, ready for attachment to bulkier groups. For all the modifications possible, the stability of the trifluoromethyl group stays a useful anchor—few functional groups hold up so well when chemists test every trick in the book.
Anyone searching for 4-Methoxy-3-Trifluoromethylbenzonitrile gets tangled in its various aliases and commercial tags. Common ones include 3-(Trifluoromethyl)-4-methoxybenzonitrile and 4-Methoxy-3-(trifluoromethyl)benzonitrile, but suppliers sometimes abbreviate or use catalog codes. Stir through patent literature, and yet another set of acronyms and numeric identifiers piles up. For anyone sourcing this chemical, awareness of these names avoids expensive ordering blunders or regulatory missteps.
Anyone who’s handled trifluoromethylated aromatics knows you need gloves, eyewear, and good airflow. Spills irritate the skin, and nitriles like this often release pungent fumes that warrant respect. Proper containment is key; trying to pipette the solid while wearing a mask that fogs up always gave me headaches. Emergency protocols in most facilities include spill absorbents for cyanide-related hazards. Local regulations may call for extra scrutiny due to potential breakdown into toxic byproducts under fire or strong acids. Shipping restrictions sometimes interrupt supply if transport companies suspect high-toxicity intermediates. I’ve seen labs sidestep a lot of woes by investing in safety training and automation—no more surprises from spilled solids in the rotary evaporator.
The influence of this molecule stretches mainly across pharmaceuticals and agrochemicals. Medicinal chemists favor it for building blocks in targeting kinases and enzyme inhibitors—its functional groups allow for targeted modification and easy metabolic tuning. Agrochemical researchers exploit the fluorinated core, which often delays bio-degradation in soil, granting these molecules a long enough half-life to be practical. 4-Methoxy-3-Trifluoromethylbenzonitrile has crossed paths with dye researchers chasing more stable colorants and specialty polymers. The bright side for researchers: suppliers now offer it in multi-gram quantities, allowing not just bench chemistry but also pilot-scale work.
Every new heterocyclic drug lead sparks another round of demand for benzonitrile building blocks. Current R&D focuses less on finding the next miracle molecule and more on increasing selectivity, solubility, and kinetic profile by altering fragments like the trifluoromethyl or methoxy positions. Structure-activity studies often run parallel—synthesizing analogs by changing the nitrile, shifting the methoxy, or swapping fluorine for heavier halides. Modern combinatorial synthesis takes advantage of reagents like this, where robust stability and versatile reactivity promote synthesis of library after library. The move toward green chemistry pushes companies to improve atom economy and energy efficiency in making this compound.
Nitriles have a checkered health record, so researchers pay close attention to toxicity screens. Acute toxicity for 4-Methoxy-3-Trifluoromethylbenzonitrile runs moderate in animal tests, but long-term studies remain sparse. Most studies point out the risk profile rises sharply with inhalation or ingestion, as rapid metabolism can unleash cyanide groups in the body. Disposal guidelines in labs draw from the same playbook as for other aromatic nitriles: avoid release into waterways, incinerate under controlled conditions, collect contaminated glassware for special handling. Large-scale industrial users sometimes deploy bioassay monitoring to avoid accidental exposure, because standard gas detection methods spot only larger molecule breakdown products. I’ve seen high-throughput screening start with benzonitrile cores, then cull candidates after quick cytotoxicity evaluations, showing just how cautious the next generation of chemical developers operates.
The continued relevance of 4-Methoxy-3-Trifluoromethylbenzonitrile owes a lot to the push for custom-designed materials and pharmaceuticals. The rising tide of fluorinated molecules in both medicine and electronics almost guarantees demand won’t fade soon. Advances in catalysis and cleaner production routes may bring down costs and reduce waste, offering both environmental and economic rewards. Automated synthesis robots and AI-driven chemical design might soon turn once-rare intermediates like this into more routine ingredients in both blockbuster drugs and high-performance materials. Researchers betting on next-level drug scaffolds or new agricultural compounds keep a close eye on every new study involving this benzonitrile, convinced the next big advance may start with a single, well-chosen fluorinated ring.
A name like 4-Methoxy-3-Trifluoromethylbenzonitrile comes off as intimidating, but if you know a little chemistry, breaking it down turns out to be pretty straightforward. The chemical formula for this compound is C9H6F3NO.
It’s easy to look at a chemical name and draw a blank, especially one as long as this. I’ve fumbled my way through countless lab reports because chemical names can feel more like alien languages than anything you’d use in conversation. The trick is to focus on the patterns. Look at the root—benzonitrile. That tells you there’s a benzene ring with a nitrile group (a carbon triple-bonded to a nitrogen), and the rest—methoxy and trifluoromethyl—are just attachments that spice up the ring.
If you scribble it out on paper, you’ll see it: benzene rings bring six carbons and five hydrogens. Nitrile swaps a hydrogen for a –C≡N group. The methoxy part means you have an oxygen and an extra carbon and hydrogen (–OCH3) sitting on the ring. Trifluoromethyl brings in three fluorines and one carbon (–CF3). Add it all up, and you get C9H6F3NO.
I remember asking myself that very question during organic chemistry. There’s a reason industry nerds care so much. If you’re working on developing a pharmaceutical drug, pesticide, or new material, a formula isn’t just trivia. It’s survival. You need to know atomic counts for safety, regulation, sourcing raw ingredients, predicting how the molecule acts in a reaction, or whether it’s going to blow up the lab given the right (or wrong) conditions.
In my lab days, a single wrong letter or number in a chemical formula meant wasted hours, wasted reagents, and sometimes a lecture from the safety officer. Precision puts food on the researcher’s table.
Coming across these tricky names, confusion breeds mistakes. Something simple like writing C9H6F3NO as C9H5F3NO means the difference between an actual existing compound and pure fiction. If distances matter in construction, atoms and bonds matter tenfold in chemistry.
That’s why chemists need better technology—smarter structure-drawing software, real-time validation tools, and clearer educational materials. Our textbooks, honestly, sometimes read like puzzle books without answers. We all could use more diagrams showing not just the formula, but how the pieces fit, much like a Lego manual. These visual aids keep students awake and steer lab techs clear of mishaps.
Building up confidence in chemical literacy starts way before college. I’ve seen high school students stare at names like “4-Methoxy-3-Trifluoromethylbenzonitrile” and feel defeated. Yet, once shown how to break down the name, count up the bits, and see how it all links together, they can figure out formulas on their own. It pushes chemistry past memorization—it’s a problem-solving game, and it’s more rewarding than I ever expected when starting out.
Asking for a formula like 4-Methoxy-3-Trifluoromethylbenzonitrile isn’t just about homework. It’s a reminder that precision matters and that making chemistry understandable matters just as much. Steps like better visuals, interactive apps, and open discussions right from school days pave the way for safer labs and sharper minds.
Most folks working in labs or small chemical plants can spot trouble when a bottle of chemical starts being stored wherever there’s space—on a sunny windowsill, in a steamy back room, or next to something flammable. 4-Methoxy-3-Trifluoromethylbenzonitrile falls into that category of specialty compounds that definitely reward a little bit of respect in storage. Mess up the basics, and not only does your stash degrade over time, you open the door to contamination, spillage, or even dangerous reactions.
If you’ve ever uncapped a bottle from long-term storage, only to find a funky layer caked on top, you already know the heartbreak of moisture sneaking in. 4-Methoxy-3-Trifluoromethylbenzonitrile, like most benzonitriles, prefers things dry. Humidity plays tricks on stability, and unseen water can trigger unwanted reactions over months. A desiccator or a tightly sealed amber glass bottle with fresh silica gel packs avoids a lot of headaches.
Ultraviolet light brings more problems than benefits in chemical storage. Over years of watching shelves, I’ve seen plenty of chemicals lose their punch, change color or even break down from sunlight. Store this compound away from direct light—think sturdy cabinets or those classic amber bottles. A dark, steady place gives you the best shot at shelf life.
Heat is the enemy of shelf life for a lot of chemicals, especially organics. A cool room—somewhere between 15 and 25 degrees Celsius—keeps molecular drama to a minimum. Colder storage often adds cost and hassle, while heat from radiators or sunny windows pushes decomposition rates up. Skip attics, furnace rooms, or anywhere that temperature swings are normal. Ordinary lab cabinets away from heat sources work just fine in most cases.
Old labels and mystery jars clutter shelves everywhere, but they’re a trap. Always label every bottle with the compound name, concentration, and opening date. Even if the bottle never moves, you’ll know whether it’s safe to use six months later. Batch numbers and supplier details keep things traceable in case regulators or safety officers come around.
Every seasoned hand knows what can go wrong when incompatible chemicals share a shelf. In a pinch, one broken bottle can mean trouble for everyone in the lab. So segregate 4-Methoxy-3-Trifluoromethylbenzonitrile from acids, strong bases, oxidizers, and reducing agents. It takes just a little planning, but nothing ruins a morning like a surprise chemical reaction.
It’s tempting to stock up, but smaller amounts go a lot further in terms of safety and freshness. You’ll use what you buy before it starts to break down, and you won’t create a hazard in case of leaks. If you work in a university or research setting, order only what you need for the next set of experiments. Unused chemicals pile up quickly, and disposal becomes its own headache.
One thing I learned from tough experiences in shared spaces: talk to your colleagues. Shared knowledge prevents repeated mistakes—from leaving a bottle open to forgetting to check for leaks. Post-up simple notes about storage procedures, and people will follow through. In the lab, a little mutual accountability beats almost any rulebook.
Sometimes it's easy to feel like storage is an afterthought, but it deserves more attention. Practicing care with 4-Methoxy-3-Trifluoromethylbenzonitrile means less waste, fewer accidents, and a better working environment for everyone involved. The right conditions don't demand fancy equipment. Just a little common sense—avoid moisture and light, stick with sturdy bottles, label everything, and stay organized. It’s the simple things that keep you out of trouble in the end.
Steps in a lab aren’t just about mixing colorful liquids in glass flasks. Sometimes, the quiet compounds work the hardest behind the scenes. Take 4-Methoxy-3-Trifluoromethylbenzonitrile. The name feels like a mouthful, but its purpose comes into play in plenty of corners of science and industry.
Drug development always needs building blocks. Chemists look for shortcuts to save weeks off synthesis. This compound shows up here as a key chunk in making complex molecules for medicines, especially in early research phases. Its structure carries a trifluoromethyl group, which tends to make molecules more stable in the body and sometimes improves their ability to reach the right spot inside us. I’ve seen lab teams choose it over simpler nitriles, just because those extra atoms in its structure tweak how a drug works.
Big pharma companies worldwide often work through libraries of related molecules, switching out small parts on a core structure. 4-Methoxy-3-Trifluoromethylbenzonitrile helps speed up that process. Companies like Pfizer and Novartis have mentioned compounds like this in their patent filings for anti-inflammatory or anti-viral drug candidates. It’s not about this molecule working as a drug itself, but about how its framework lets scientists rapidly build better medicines.
It’s no secret that crops face threats from pests and fungi. Modern farming doesn’t only use old-school chemicals like DDT—scientists keep searching for new ways to target pests with fewer side effects. The structure of 4-Methoxy-3-Trifluoromethylbenzonitrile fits snugly in this role, letting chemical engineers string together more elaborate pesticide ingredients.
Trifluoromethyl groups increase power and longevity in fields. So, when agrochemical researchers need something persistent but efficient, they often reach for intermediates like this. Talking with folks in herbicide and fungicide R&D, it's clear: unique nitrile-containing aromatics shorten the route to making safer, more selective molecules. They’ve helped bring down the amount of chemical needed per acre, cutting costs and environmental load.
Chemists know the value in structure—swap a single atom, the color or function of a material shifts. Aromatic nitriles like this one contribute in specialty plastics and dyes, where precision shapes how a product performs. For heat-resistant polymers or high-performance coatings, a trifluoromethyl group offers durability against acids, alkali, and sunlight. Paint manufacturers, seeking colors that don’t fade in the sun, use these kinds of compounds to hold dye molecules steady year after year.
Some of the boldest industrial dyes used in electronics or solar panels link to this chemistry. In screen technology, you don’t want a TV’s vibrant panel turning dull after a summer in a sunny window—each tweak in the chemical backbone boosts lifespan and keeps displays bright.
Not all uses come without headaches. Trifluoromethyl chemistry demands smart handling, as these groups can stay stubbornly in the environment. Some labs recycle solvent streams and tighten up their reaction recipes to minimize waste. Green chemistry experts push suppliers to deliver cleaner versions and shorter synthetic pathways, so less ends up downstream. At industry conferences, there’s excitement about using catalysts and renewable inputs to reduce the environmental footprint along the way.
Real progress comes from open conversation between scientists, farmers, and environmental groups. With enough transparency, we can keep the practical applications while making the production process safer for waterways and workers. The story of 4-Methoxy-3-Trifluoromethylbenzonitrile shows how small molecules shape big industries, but it’s up to all of us to make sure the benefits outweigh the cost.
People often believe that working in a lab feels a bit like preparing a fancy meal—lay out your ingredients, follow your recipe, keep your space tidy. The main difference? A misstep in the lab goes far beyond a burnt dinner. Chemicals like 4-Methoxy-3-Trifluoromethylbenzonitrile serve as a clear example. It’s a mouthful, sure, but don’t let the odd name downplay the responsibility that comes with it. If you encounter this substance in a work setting, safety matters more than ever.
I’ve spent plenty of days handling fine powders and eye-watering liquids, knowing well how quickly a minor accident can pop up. 4-Methoxy-3-Trifluoromethylbenzonitrile gets its danger from its structure. Compounds with a benzonitrile backbone often hit the warning lists for toxicity. This one even brings along a trifluoromethyl group—an addition chemists prize for making molecules more stable in a reaction, but it doesn’t make the chemical any kinder to lungs or skin. Such substances tend to irritate eyes, skin, and upper airways. Some can sneak right through skin, reaching the bloodstream without much resistance. Sudden headaches, coughing fits, or angry red skin don’t leave much doubt when exposure happens. Based on data from chemical suppliers and open safety databases, this compound lands in the “harmful on contact, inhalation, or ingestion” group. That raises concerns for anyone handling it—seasoned chemist or new technician alike.
I’ve watched colleagues shrug off goggles or delay glove changes just to hurry things along. It’s easy to think, “One quick transfer, nothing will go wrong.” Those shortcuts add unnecessary risk. Safety data sheets don’t mince words about the need for goggles, gloves resistant to chemical penetration, and well-fitted lab coats. Bad or careless technique catches up eventually—either through minor rashes today or long-term health problems down the road. The right gear saves more than doctors’ bills. Chemical-resistant gloves keep your hands out of trouble. Splash goggles block those accidental squirts. A fume hood carries away any vapor that might otherwise linger just long enough to hurt someone.
Keeping a workplace safe depends far less on high-tech gadgets and far more on behavior. Anyone who has ever trained a new lab member knows habits form fast. Eyes on task, double-check those labels, store waste where the law requires, clean up right away. These steps don’t just protect the person taking them; they protect everyone in the room. It makes sense to store 4-Methoxy-3-Trifluoromethylbenzonitrile in tightly sealed containers, away from both heat and sunlight. Proper labeling isn’t just red tape—it means someone else won’t stumble into the same hazard later.
It helps to build a culture where safety isn’t an afterthought at the end of a busy day. Safety meetings where people share small mishaps help everyone get smarter. Posting clear instruction sheets at eye level and checking safety equipment every month makes preparation part of the routine. Emerging digital tools, like online inventories and digital checklists, hold workers accountable in a way paperwork can’t always match. Proactive steps create a safer environment—not just for now, but for all those students, technicians, and researchers who walk in later. In the end, staying humble in the face of chemicals keeps everyone healthy—no shortcuts, no excuses.
Browsing through chemical catalogs, some numbers jump out. Purity isn’t just a technicality in the world of building blocks like 4-Methoxy-3-Trifluoromethylbenzonitrile—it’s the difference between a clean synthesis and a reaction that throws scientists back to square one. Most suppliers stamp this compound with a minimum of 98% purity. Some bump it up to 99%, but that usually brings a heavier price tag. For folks in organic chemistry or those scaling up from lab to industrial batch, purity isn’t just about avoiding hassle; it means knowing what’s really in your flask.
One lot from a big-name supplier comes with a printout: “Purity: ≥98.0% (GC)”. That GC, that's gas chromatography. It means the bulk of what's in the bottle is your target compound, and only a sliver—no more than two percent—is some mix of related organics, water, or trace residues. In other cases, the certificate of analysis gets more granular. Some labs care about the moisture content, heavy metals, or halide impurities. A high-end spec sheet could show water below 0.2% by Karl Fischer, and an internal standard confirming no oddballs beyond the detection limit.
Imagine years of research getting tripped up by an unknown contaminant. Any organic chemist will tell you, extra stuff in a bottle can change color, throw off yields, and throw spectrums out of whack. For pharma, a couple percent off-black sheep feels catastrophic. These narrow specs aren’t just bureaucracy—they’re guardrails. Fail to hit those numbers and batches might fail downstream, burning weeks and budgets.
Not every lab writes home for 99.9% purity. In medicinal chemistry or early process development, 98% looks good enough. As the process gets tighter, those cutting-edge teams start shopping from premium lines, hoping for fewer headaches. Sometimes, they give up a chunk of sample for their own NMR check. You pay more for that peace of mind—sometimes double.
Here’s one reality: chemical plants rarely churn out pristine, single-compound streams in one go. Maybe the answer sits with better purification: a second round of column chromatography, a sharper distillation, or recrystallization. Other times, companies could offer batch-specific data, letting customers check details before purchase rather than gambling on a label.
Suppliers might roll out more transparency through batch tracking and make high-purity lots an upcharge rather than a gamble. For buyers, running an HPLC or NMR quickly on arrival keeps nasty surprises at bay. A chemical doesn’t become better because the catalog says so—good labs always trust, but verify.
Customers don’t buy fancy-sounding thresholds for the fun of it. If a pilot synthesis keeps stalling, tweaking the purity of 4-Methoxy-3-Trifluoromethylbenzonitrile might fix more than expected. Verified specs are more than marketing—they’re a bet against wasted time. Keeping one eye on impurity limits turns into less guesswork across the bench and a smoother run, batch after batch.
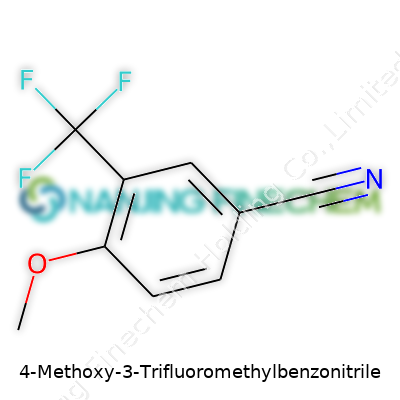

| Names | |
| Preferred IUPAC name | 4-methoxy-3-(trifluoromethyl)benzonitrile |
| Other names |
4-Methoxy-3-(trifluoromethyl)benzonitrile 3-(Trifluoromethyl)-4-methoxybenzonitrile 4-Methoxy-3-(trifluoromethyl)benzene-1-carbonitrile 4-Methoxy-3-trifluoromethylbenzonitrile |
| Pronunciation | /fɔː ˈmɛθ.ɒk.si θriː traɪˌfluː.rəˈmɛθ.ɪl ˌbɛn.zəʊˈnaɪ.trɪl/ |
| Identifiers | |
| CAS Number | 328-86-3 |
| Beilstein Reference | 1599435 |
| ChEBI | CHEBI:77911 |
| ChEMBL | CHEMBL3707081 |
| ChemSpider | 23419423 |
| DrugBank | DB08335 |
| ECHA InfoCard | 01-2120120097-60-0000 |
| Gmelin Reference | Gmelin Reference: 84113 |
| KEGG | C18709 |
| MeSH | C10H6F3NO |
| PubChem CID | 1209365 |
| RTECS number | GZ1152000 |
| UNII | UR784O6P1V |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C9H6F3NO |
| Molar mass | 185.15 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.29 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 0.0182 mmHg at 25°C |
| Basicity (pKb) | pKb = 9.7 |
| Magnetic susceptibility (χ) | -61.06·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.504 |
| Dipole moment | 3.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 331.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -211.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | no data |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0-☢ |
| Flash point | Flash point: 113.8°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | DH2302000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Methoxy-3-Trifluoromethylbenzonitrile is not established. |
| REL (Recommended) | 10g, 25g |
| IDLH (Immediate danger) | Not listed |