The story of 4-Amino-3-Chloro-2-Fluorobenzonitrile ties back to the chemical industry’s ongoing search for new molecular building blocks in pharmaceutical and agrochemical research. Going back several decades, scientists started modifying aromatic rings by hanging groups like fluoro, chloro, amino, and nitrile onto different positions, revealing unique reactivities and applications. Chemists want new core structures that can tune biological activity or unlock new types of chemical reactivity. That’s where this molecule fits. In research groups and R&D labs around the world, it’s gained a following as a flexible intermediate. The adoption of halogenation chemistry in the 20th century, bolstered by better manufacturing safety, set the stage. Fluorine chemistry once caused hesitation due to toxicity and technical hurdles, but by the 1990s, patience and clever engineering tamed much of the risk, expanding choices for what could be practically produced in kilogram and ton batches.
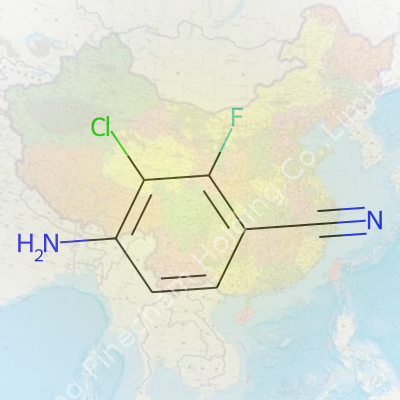
4-Amino-3-Chloro-2-Fluorobenzonitrile shows up as a pale solid, catching little attention on the bench, but in practice offers all kinds of routes for downstream synthesis. Chemists know it as a niche benzene derivative offering a rare pattern of substitution: one side fits a strongly electron-withdrawing nitrile, balanced by both a fluoro and chloro atom that bring their own tricks for selectivity or further modification. The amino group, always a useful handle, sits ready for coupling or protection. Its chemical formula, C7H4ClFN2, keeps things compact, and the molecular weight lands at 170.57 g/mol. Suppliers often keep it in stock for medicinal and process chemistry, recognizing the demand comes from hardheaded problem-solving rather than volume use.
Handling 4-Amino-3-Chloro-2-Fluorobenzonitrile doesn’t call for unusual gear, but being mindful never hurts. The compound sits as a solid at room temperature, and the melting point tends to cluster around the 90–95°C mark. Its solubility profile matches most benzonitriles: sparing in water but dissolving reasonably well in common organic solvents like DMF, DMSO, and dichloromethane. The combination of an electron-poor nitrile and electron-rich amino gives it unique reactivity, especially in cross-coupling or nucleophilic aromatic substitution. A faint odor sometimes leaks out on opening the bottle, not altogether pleasant but not overpowering either, which happens more often with halogenated aromatics. Stability holds up under standard storage—so long as exposure to moisture and heat is minimized, the powder remains unchanged on the shelf for years.
Quality speaks loudest in research chemicals, and here, assay values above 98% are the expectation. Impurity profiles usually highlight trace isomers or residual solvents, and reputable suppliers back up claims with HPLC, NMR, and MS data. CAS registry, 86483-91-4, tracks it in inventories worldwide. Labels warn of potential respiratory irritation, skin contact issues, and call for handling in a fume hood. Batch numbers trace everything back for recalls or quality concerns. Most containers stick to small glass bottles sealed against air and light, though commercial orders sometimes use foil-lined drums with nitrogen backs to prevent oxidation or hydrolysis. Proper labeling matters because a mix-up with benzonitrile derivatives can throw off a whole reaction sequence, burning precious time and resources.
Making 4-Amino-3-Chloro-2-Fluorobenzonitrile takes some experience. The typical route starts from a multi-substituted benzene, often 3-chloro-2-fluorobenzonitrile, and then brings in the amino group using direct amination—usually with ammonia under pressure, a catalyst like palladium, and careful control of temperature. Sometimes, chemists opt for stepwise protection and deprotection depending on desired purity and the scale of synthesis. Other routes might install halogens later, using directed ortho-metalation followed by halogenation and Sandmeyer-type reactions, but this approach chews up more time and materials. Yields sit in the moderate range, typically 60–80%, with most failures tracing to impure starting materials or over-alkylation at the amino site. Scale-up to pilot plant volume calls for sharp focus on reaction exotherms and gas handling, and always, thorough cleaning to keep cross-contaminants at bay.
The real magic of 4-Amino-3-Chloro-2-Fluorobenzonitrile shows once it hits the reaction flask. That free amino group opens doors to acylation, sulfonation, or even urea formation. Medicinal chemists exploit it for making heterocyclic cores—a move into pyrazoles, triazines, and fused ring systems. The chloro and fluoro swap out under the right conditions in nucleophilic aromatic substitution, especially with electron-rich nucleophiles like alkoxides or thiolates. Some groups use the nitrile for further extension—hydrolysis gives amides or acids, reduction moves to amines, and Grignard addition goes toward ketones. This versatility lets researchers dial in exact properties in drug candidates or crop protection agents. Every new derivative brings a slight shift in lipophilicity, electron density, or hydrogen-bonding, letting chemists fine-tune for specific biological targets or formulation strategies.
Anyone searching product catalogs for this molecule bumps into a jungle of alternative names. Aside from “4-Amino-3-Chloro-2-Fluorobenzonitrile,” suppliers might call it “2-Fluoro-3-chloro-4-aminobenzonitrile,” “4-CN-3-Cl-2-F-aniline,” or “Benzonitrile, 4-amino-3-chloro-2-fluoro-.” The IUPAC systematic name rarely appears on bottles but sometimes in regulatory paperwork: “4-amino-3-chloro-2-fluorobenzenecarbonitrile.” The molecular shorthand C7H4ClFN2 and CAS number 86483-91-4 serve as the gold standard in keeping things straight—especially helpful when dealing with custom synthesis companies or navigating customs for imports and exports.
Working with 4-Amino-3-Chloro-2-Fluorobenzonitrile reminds any lab hand that halogenated aminobenzonitriles deserve respect. Splashing powders or vapors into eyes or lungs turns unpleasant fast, with irritation and inflammation showing up at low exposure. Gloves, goggles, and proper ventilation set the baseline. Handling waste means separating halogenated organics for incineration or designated chemical recycling—not a compound to flush or toss in general trash. Even for experienced chemists, double-checking MSDS sheets before scaling up stays smart, since the blend of amino, fluoro, chloro, and nitrile can spark allergic or sensitization reactions for those repeatedly exposed. In production, process safety teams track potential for runaway reactions—especially in the amination and halogenation steps—and lock in procedures for spills, evacuations, and inerting.
Most demand for 4-Amino-3-Chloro-2-Fluorobenzonitrile arises from life sciences, especially drug discovery and agrochemical leads. Its unique substitution pattern lines up well for SAR (structure-activity relationship) expansions, where medicinal chemists look for minor tweaks that boost potency or cut side effects in clinical candidates. In crop science, it sits as an intermediate for fungicides, herbicides, and insecticides that target resistant strains. Material scientists occasionally grab for it in specialty polymers or liquid crystals, chasing specific optical or electronic properties. The molecule’s unpredictability sometimes frustrates newcomers but draws repeat attention from research teams solving real synthetic puzzles.
R&D efforts around 4-Amino-3-Chloro-2-Fluorobenzonitrile reflect a drive for efficiency and innovation. Industrial groups tweak routes to boost purity, yield, and safety, shaving time and cost. Universities dig into its reactions with novel catalysts, hoping to unlock milder conditions or unlock regioselectivity heretofore undiscovered. There’s steady activity probing the biological activity of derived systems—antiviral agents, kinase inhibitors, and new pesticides roll out in the literature. Patent filings track shifts in demand, with a spike whenever new disease threats or resistant agricultural pests hit the news. The constant push for greener, less wasteful methods means biocatalytic steps and flow chemistry appear in recent papers, aiming to sidestep toxic reagents or bulky waste streams that are harder to manage in today’s regulatory climate.
Questions about toxicity keep coming up, both for workers and end-users. Animal models suggest low-to-moderate acute toxicity, with risks climbing sharply at higher exposures. The combination of halogen atoms and aromatic nitriles historically raised red flags for potential mutagenicity or environmental persistence, so ongoing work aims to clear up the long-term risks. Standard practice calls for pre-screening all new derivatives against a panel of safety assays, including AMES, hERG binding, and aquatic toxicity screens. Published data show most handling incidents trace back to lax PPE use rather than inherent danger, but regulatory agencies watch closely, especially as research moves toward consumer-facing drugs or agricultural products. Degradation studies point to modest stability in soil and water, but questions linger around breakdown byproducts and their bioaccumulation potential, issues anyone in industrial scale-up needs to answer before moving ahead with market introductions.
The chemical space surrounding 4-Amino-3-Chloro-2-Fluorobenzonitrile keeps growing, driven by the hunt for better therapies and environmentally safer crop tools. With the ever-tightening rules on chemical safety and emissions, demand grows for production that cuts waste and limits toxic byproducts. Advances in continuous-flow reactors, greener solvents, and catalytic process design respond to these pressures, making preparation safer and more efficient every year. Novel derivatives, often with minor tweaks to the core, roll out for testing in anti-cancer, antiviral, and resistance-breaking crop protection pipelines. Some researchers screen entire libraries derived from this scaffold against big data-driven targets in hopes of finding a surprise hit, while others refine known reactions to lower costs. Regulations will get tighter, but chemistry rarely stands still for long—the simple act of putting the right groups on the right ring keeps unlocking new opportunities, and 4-Amino-3-Chloro-2-Fluorobenzonitrile stands as proof that even modest building blocks can open a path to bigger discoveries.
Every now and then, a mouthful of a name like 4-Amino-3-Chloro-2-Fluorobenzonitrile lands on my desk. Stripping away the jargon, what you find under the hood is a compound with the formula C7H4ClFN2. Seven carbon atoms, four hydrogens, one chlorine, one fluorine, and two nitrogens. Chemists arrange these atoms around a benzene ring, marking off spots for each additional group.
People gravitate toward this information for solid reasons. Pharmaceutical developers and material chemists need to know the formula before running any calculations. And if you have ever played with synthesis planning, you know any mistake on this front throws the whole result off. It’s easy to underestimate the value that comes from just writing things clearly, but in a pinch, the right formula saves days if not weeks of troubleshooting.
Now for the molecular weight: 170.57 g/mol. Put simply, this tells you what one tiny mole of the stuff would weigh out on a scale. We all remember those endless labs in high school, searching for exact weights because the recipe called for precision. Most work in R&D labs requires careful weighing; without knowing the true molecular weight, preparing accurate solutions doesn’t happen.
I remember a time struggling with stubborn nitriles. Wrong molecular weights cost us a full week and left us with nothing but a weird tar that wasn’t even close to the planned product.
Seeing numbers and strange ringed diagrams, a lot of people zone out, assuming it doesn’t touch their daily lives. That’s not my experience. These are the building blocks that shape entire sectors. In pharmaceuticals, a slight mistake in a formula means a failed clinical trial or a safety risk. In agriculture or advanced materials, these subtleties dictate how tough a new compound works in field trials. Every chemical structure on a patent protects an innovation, not just for who made it, but for anyone who wants to avoid infringing.
Regulatory groups need exact details too. Before any company can test or import new chemicals, toxicology teams check formulas and weights, running models to flag hazards or approve production. Fumbling a number or symbol could make all the work irrelevant or even illegal. Safety data relies on this. Compliance with global rules—from Europe’s REACH system to the US EPA—pivots on the right information.
Not everyone needs to get deep into molecular diagrams, but everyone leans on accurate data. Tech tools help, but nothing replaces going line-by-line through a formula and double-checking. Chemists and lab techs still keep the habit of jotting details on paper, even in an era when databases spit out information in seconds. Thinking critically about formulae and weights grounds each project, whether it’s a huge factory batch or a single gram for lab experiment.
Better access to this kind of precise data cuts mistakes and sharpens thinking across industries. Encouraging openness and transparency in chemical databases reduces easy errors and makes it harder to hide behind poor recordkeeping. For anyone facing new chemical syntheses, scouring for the right formula and molecular weight is the smart place to start.
Chemistry always felt distant in high school, but the world runs on compounds with names that don’t exactly roll off the tongue. 4-Amino-3-Chloro-2-Fluorobenzonitrile is one of those odd-sounding chemicals, and yet, its real-world impact slips quietly into the background of everyday life. Behind the scenes, compounds like this help drive important progress in various industries, especially pharmaceuticals and agriculture.
Touching on the personal impact, I’ve seen drug innovations over the years transform how families handle chronic illnesses. What often gets missed is the pipeline behind these new pills and injections. This particular compound stands at an early leg of that journey. It pops up in the synthesis of advanced molecules — basically, scientists use it as a starting block to build more complex chemicals for treatment options. In drug labs, these sorts of chemicals let researchers add groups like fluorine or chlorine in specific spots, which can mean a drug gets absorbed better or fights bacteria harder.
For example, plenty of cancer drugs and antibiotics start from scaffolds that look a lot like this one. The goal isn’t to swallow 4-Amino-3-Chloro-2-Fluorobenzonitrile itself, but to tweak molecules so the final medicine becomes safer and more powerful. Without relatively simple building blocks, these improvements usually cost more, take longer, and—frankly—risk a lot more dead ends before any hope of actual treatment.
Farmers battle fungus and bugs every single growing season. I grew up in a little town ringed by cornfields, and half the spring school lessons got interrupted by news of new pesticides or resistant bugs. Modern crop chemicals rely more and more on molecules built from complex aromatic rings—basically, shapes with benzene and pieces like nitriles or amino groups. This helps keep costs down for farmers who need the latest pesticide generation to work on crops without nuking the soil.
Companies build just about every new generation of fungicides and insecticides off intermediate chemicals. The subtle mix of chlorine and fluorine changes how long the spray sticks around, whether it runs off with the rain, and how much residue ends up inside the plants. 4-Amino-3-Chloro-2-Fluorobenzonitrile opens up creative paths for researchers, speeding up new products that sometimes stand between a good harvest and total crop failure.
Here’s the rub—working with specialty chemicals brings health and safety headaches. In my student lab days, handling nitriles meant masks, gloves, and waste containers labeled with enough warnings to spook anyone. Many intermediates turn nasty with just enough bad handling. Factories produce them in batches that need careful oversight to avoid spills or leftover byproducts that can make their way into water supplies.
Cleaner manufacturing methods are slowly finding space in the industry. Companies now test alternative catalysts and look for greener solvents to limit waste during synthesis. Seed grants for small research labs, tougher environmental audits, and closer partnerships with regulatory teams can help shrink the risk. It’s not about pulling the plug on complex chemistry, but making sure labs—big or small—stick to safer habits. This also means consumers and farmers keep benefiting from scientific advances, without picking up the tab in their local water supply or hospital visits due to mishaps in handling.
Chemicals like 4-Amino-3-Chloro-2-Fluorobenzonitrile show up in tricky places: medicine cabinets, food chains, and even rivers. Their best uses mean fewer side effects in drugs, less harm to bees and frogs after a spray, and more control over the path from lab bench to real-life results. Life gets better when both industry and regulators share the table, challenge old habits, and open the door to new ideas—one complicated compound at a time.
Walk into any research lab, and you’ll find shelves lined with carefully labeled bottles—each one expecting the right care to stay safe and useful. Take 4-Amino-3-Chloro-2-Fluorobenzonitrile as an example. This isn’t just another chemical name to gloss over; the way folks store this stuff can shape both lab safety and research outcomes in a pretty big way.
Labs often get careless with “routine” materials, forgetting that some compounds sneak in extra hazards. 4-Amino-3-Chloro-2-Fluorobenzonitrile brings together reactive amine, halide, and nitrile groups. If you stack the bottles too close to heat, moisture, or dust, subtle changes can mess with purity—or worse, trigger irritation in the air if a cap’s left loose. I’ve seen technicians rush and drop a sample near an air vent, and within a day, traces of degradation show up when purity testing rolls around.
Safety crews post clear recommendations for a reason. Common sense tells us dry, cool, well-ventilated spaces trump a sunlit shelf every time. Direct sunlight or warmth nudges chemicals toward breakdown. I remember one summer, the air conditioner failed for a weekend. Several heat-sensitive reagents—including a related aromatic nitrile—showed crystal discoloration after just two days.
For 4-Amino-3-Chloro-2-Fluorobenzonitrile, stick to a temperature below 25°C (about 77°F), out of direct sunlight or bright indoor lighting. Even small heat spikes speed up slow reactions you’d rather avoid. Humidity sneaks in and hydrolyzes sensitive groups, so a desiccator or a cabinet with silica gel packs works best. Seals matter—a tight cap saves headaches down the line.
Ventilation matters, too. The compound isn’t super-volatile, but dust or accidental spills become a problem fast without good airflow. I’ve worked in spaces where minor irritants quickly became miserable to be around, just because the air sat still and fumes built up.
Part of thoughtful storage means never forgetting exactly what’s in each bottle. Faded, peeling labels invite mix-ups—including situations where unwanted chemical reactions turn small mistakes into facility-wide problems. Using sturdy, legible labels and double-checking the date and content before shelving make a difference.
Organizing storage also cuts down the odds of accidental cross-contamination. If you keep oxidizers, acids, and organic reagents all together, sooner or later someone knocks the shelf, and you’re suddenly mixing things you absolutely shouldn’t. Keeping this compound away from strong acids, oxidants, and other reactive groups avoids these headaches.
Automated monitoring tools, simple temperature logs, and regular audits sound old-fashioned, but in practice, they keep people honest. I’ve watched a technician quietly fix a too-warm cabinet after a digital alert pinged their phone. It’s easy to think regular checkups take too much time, but cleanup from a contaminated or degraded batch takes a lot longer.
People often underestimate the value of clear communication between shifts too. Sharing reminders about which chemicals have stricter storage rules builds good habits and helps new team members avoid rookie mistakes.
Caring for a chemical like 4-Amino-3-Chloro-2-Fluorobenzonitrile isn’t just about ticking off storage requirement lists—it’s a piece of long-term science and health. Small steps—keeping things dry, cool, sealed, clearly marked, and well-separated—keep everybody safer, protect research results, and cut down wasted time and resources. That’s a lesson that sticks, lab after lab.
Anyone dealing with chemical products, pharmaceuticals, or food additives knows that purity isn’t just a fancy number stamped on a certificate. Years ago, while working in a quality assurance role, I learned pretty quickly how a small difference in purity levels could sway a customer’s trust or disrupt an entire production run. For a lot of products, purity specs go way beyond marketing claims; they serve as a core promise. Sometimes you see 98%, 99.5%, or USP-grade; these are anchored in real tests and daily practice. Customers don’t just look at the percentage—they worry about what the remaining fraction holds. Trace metals, environmental contaminants, or moisture content get flagged right away during audits. In pharmaceuticals, even a 0.1% off-spec can lead to regulatory problems. A colleague once caught a batch with slightly higher impurities; the fallout included hours of extra paperwork and, worse, a delay in supplying hospitals.
For food ingredients, buyers often want certificates that break down individual impurity levels, not just a headline percentage. Having spent late nights reading through third-party lab reports, I know how even minor deviations can mean withdrawing an entire shipment or launching a costly investigation. Purity matters to every link in the supply chain—farmers, manufacturers, distributors, and ultimately, the end user. It shapes shelf life, taste, and even safety. In the years I spent tracing quality complaints, most issues had roots in misunderstood or misstated purity numbers.
Talking about packaging sizes might sound trivial at first, but every plant manager or small business owner knows the headache that comes from poor choices here. Once, I watched a 25-kilo bag rip open in transit, spilling powder everywhere—and the subsequent clean-up chewed through time and tempers. Most vendors offer their standard packages: bottles for the lab (sometimes just 100 grams or a few milliliters), pails or drums for bulk buyers (like 5, 10, 25, or 50 kilograms), and custom containers for larger orders.
Smaller packaging isn’t just about convenience for lab testing. Some customers—bakers or supplement brands—prefer smaller containers to avoid spoilage or caking. At the other end, chemical plants want bulk drums or even totes, and they worry non-stop about moisture ingress or contamination during repackaging. Working with a textile plant last year, I learned that switching from 25-kilogram bags to 1,000-kilogram supersacks required revamping both handling equipment and employee training.
Getting packaging right makes or breaks efficiency in a warehouse. Skids of small boxes tie up more pallet space, whereas poorly sealed bulk drums drive up waste costs. I remember tracking a customer complaint because the packaging film allowed just enough air through to ruin a high-purity batch by the time it arrived. Producers who respond to requests for custom packaging—like vacuum-sealed or inert-filled pouches—get repeat business because buyers feel listened to and protected.
It’s easy to focus on testing or marketing, but there’s real value in tightening specs and listening to real-world users about packaging. More companies share batch-level lab reports online now, making life easier for customers who don’t want to wait days for answers. Some forward-thinking suppliers work closely with logistics teams to test new packaging and actually use customer feedback, rather than relying on old assumptions. When these updates filter through, everyone along the chain—producer, buyer, transporter, retailer—deals with fewer surprises, fewer losses, and a lot more trust.
Working in a chemical lab always comes with risks, and every compound brings its own quirks. Take 4-Amino-3-Chloro-2-Fluorobenzonitrile as an example. It turns up in research benches and industrial projects, often used as a building block in pharmaceuticals or specialty materials. People might call its name unfamiliar, but its risks are the same as many other nitrile derivatives—caution, proper handling, and respect for personal protective gear sit front and center once the bottle is open.
Forget about half-measures. Standard lab coats, nitrile gloves, and snug-fitting goggles are the minimum. This compound can irritate the skin or eyes, and its dust shouldn't end up in anyone’s lungs. Inhaling fine powders rarely leads to anything good, especially with halogenated benzonitriles. Fume hoods aren’t just bench decorations—they suck up vapors and help keep noses clear from unwanted chemical sting.
4-Amino-3-Chloro-2-Fluorobenzonitrile stays happiest in a tightly sealed container, tucked away from heat, moisture, and sunlight. Humidity messes with the stuff, potentially leading to unexpected hydrolysis or breakdown products. Even the most organized folks can slip up if labeling isn’t clear—as someone who’s seen mystery jars cause full lab evacuations, there’s little room for error. Proper labeling avoids mix-ups, and, more importantly, accidental exposure.
Ventilation factors into safety just as much as gloves or lab coats. Working with chemicals like this outside a fume hood boosts the risk of accidental inhalation or spreading dust across shared surfaces. I remember an incident where dust from a chemical—different nitrile, similar risks—settled where least expected, resulting in a whole afternoon of cleaning and delayed work. That's frustration that no one needs, all avoidable with a working hood and a clean workspace.
Chemical spills shut down routines and add new entries to to-do lists. For spills involving 4-Amino-3-Chloro-2-Fluorobenzonitrile, absorbent pads and proper containment take priority right away. Skin contact should never be shrugged off; any exposure should lead to thorough washing and, if necessary, medical help. Carrying spill kits stocked with gloves and absorbent materials keeps accidents from turning into bigger problems. Disposal also matters. Pouring this compound down the drain risks both safety and environmental harm. The right move is collecting waste and handing it off as hazardous material, ticking all the regulatory boxes.
Technologies evolve, and so do the best practices for handling chemicals. Safety showers, accessible eyewash stations, and constant refresher training protect people, not just property. Even with written safety guidelines, nothing replaces a practiced routine, like knowing exactly where the eyewash sits or how to don a respirator mask. Small habits lead to fewer close calls. In my experience, complacency or shortcuts usually catch up—sometimes fast, sometimes slow, but rarely without a lesson.
Choosing to work with 4-Amino-3-Chloro-2-Fluorobenzonitrile asks for more than ticking boxes on a checklist. Proper gear, disciplined habits, and a culture that doesn’t brush off “minor” risks build safer labs. As new tech and regulations develop, these basics will still anchor the safe use of chemicals both new and old.
| Names | |
| Preferred IUPAC name | 4-amino-3-chloro-2-fluorobenzene-1-carbonitrile |
| Other names |
3-Chloro-4-amino-2-fluorobenzonitrile 2-Fluoro-3-chloro-4-aminobenzonitrile 4-Amino-2-fluoro-3-chlorobenzonitrile |
| Pronunciation | /ˈfɔːr əˈmiːnəʊ ˈθriː klɔːrəʊ ˈtuː ˈflʊə.rəʊ bɛnˈzoʊ.nɪˌtraɪl/ |
| Identifiers | |
| CAS Number | 543728-72-9 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4-Amino-3-Chloro-2-Fluorobenzonitrile**: ``` NC1=CC(Cl)=C(C#N)C=C1F ``` |
| Beilstein Reference | 3908554 |
| ChEBI | CHEBI:141442 |
| ChEMBL | CHEMBL3692968 |
| ChemSpider | 16072610 |
| DrugBank | DB08338 |
| ECHA InfoCard | ECHA InfoCard: 100.195.427 |
| EC Number | 875781-18-1 |
| Gmelin Reference | 787376 |
| KEGG | C19753 |
| MeSH | D000077209 |
| PubChem CID | 1265952 |
| RTECS number | **BZ7590000** |
| UNII | 9K250ZL33F |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | OVS70281V7 |
| Properties | |
| Chemical formula | C7H4ClFN2 |
| Molar mass | 170.57 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 1.7 |
| Vapor pressure | 0.00382 mmHg at 25°C |
| Acidity (pKa) | 14.77 |
| Basicity (pKb) | pKb = 6.06 |
| Magnetic susceptibility (χ) | -74.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.585 |
| Dipole moment | 3.58 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 183.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -46.1 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS06,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261-P280-P305+P351+P338-P337+P313 |
| Lethal dose or concentration | Oral rat LD50 > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | NA23 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg |
| Related compounds | |
| Related compounds |
2,4-Dichloro-3-fluorobenzonitrile 2-Fluoro-4-nitrobenzonitrile 4-Amino-2-fluorobenzonitrile 3-Chloro-2-fluorobenzonitrile 2,4-Difluoro-3-chlorobenzonitrile 4-Amino-3-chlorobenzonitrile 4-Amino-2-chlorobenzonitrile |