Organic chemists started showing real interest in substituted benzonitriles once the pharmaceutical and agrochemical industries realized that small tweaks to an aromatic ring could lead to big gains in potency and stability. The world has a knack for finding unique uses for fluorinated aromatics. Decades ago, 3-Methoxy-5-Trifluoromethylbenzonitrile barely made a blip in catalogs. Over time, as cross-coupling methods improved and the demand for unique trifluoromethyl structures picked up, this compound carved out its own niche. Synthetic methods moved forward along with the tools. Researchers who once wrestled with messy purification steps soon found comfort in more reproducible reactions, which changed not just how this molecule was viewed, but also how widely it would be used across industries.
3-Methoxy-5-Trifluoromethylbenzonitrile grabs attention thanks to its three functional groups: an electron-withdrawing trifluoromethyl group, a polar cyano group, and a methoxy moiety. Companies list it under varied catalog numbers, and it generally shows up as a colorless to pale yellow solid. This chemical’s appeal shines through in its versatility, forming the backbone in making everything from pharmaceutical intermediates to specialty monomers. Every batch comes with specifics, ranging from purity claims (98% or greater) to handling recommendations because experienced chemists know that even small impurities can ruin an entire synthesis.
This compound’s melting point clusters between 60°C and 65°C, but anyone storing it for longer periods should watch for subtle discoloration as an early warning of degradation. Its moderate solubility in polar organic solvents like acetonitrile and dichloromethane opens up doors for both solution-phase synthesis and purification. The electron-poor aromatic ring shrugs off many kinds of electrophilic substitutions but stays active toward nucleophilic aromatic substitution if reaction conditions get harsh. The trifluoromethyl group brings with it not just bulk, but strong electron-withdrawing power, which affects both reactivity and metabolic resistance.
Lab suppliers and manufacturers provide a CAS number (231294-37-8), molecular formula (C9H6F3NO), and common abbreviations right on their bottles. Labels include hazard pictograms for acute toxicity and eye irritation, as anyone in a real chemistry lab would demand. Shelf-life information may not appear unless specifically requested, which can catch unwary new users off guard. The technical sheet or safety data sheet clarifies purity standards, recommended storage temperatures (often cool, dry, away from sunlight), and guidelines for avoiding contact with incompatible chemicals like strong acids or bases.
Synthesis typically pivots on the sequence—placing each group on the aromatic ring without running afoul of unwanted byproducts. Most labs build the trifluoromethyl group onto a methoxybenzonitrile scaffold using copper-mediated trifluoromethylation, or they come at it from a different angle by starting with the trifluoromethyl benzene and introducing the nitrile group through Sandmeyer conditions followed by methoxylation. Some retrosynthetic routes prefer Grignard chemistry for site-specific manipulation, but what matters in the real world is yield and scalability. Purifying the product—through column chromatography or crystallization—separates the careful operator from amateur attempts, especially at larger scales.
The cool thing about this molecule is seeing how each functional group shapes its destiny. The methoxy group activates the aromatic ring for directed metalation, opening modification at the ortho position. Experienced chemists use the cyano as a springboard, transforming it under basic or nucleophilic conditions into amides, carboxylic acids, or other valuable motifs. The trifluoromethyl group doesn’t just sit there; it modifies the ring’s electronic characteristics so much, the whole molecule shrugs off attempts at traditional electrophilic bromination or sulfonation. This pattern forces chemists to rethink reaction strategies, often switching to transition-metal catalysis or other modern tools to unlock specific modifications.
Catalogs list 3-Methoxy-5-Trifluoromethylbenzonitrile under several guises: MFBN, 3-Methoxy-5-(trifluoromethyl)benzonitrile, and sometimes just as its CAS number. Different companies prefer proprietary branding, which sometimes causes confusion when hunting down literature references or comparing prices. Synonyms matter most during ordering or literature searches, as mismatched names can trip up procurement teams and delay research projects.
Working safely with this chemical takes more than reading a safety data sheet—it means understanding the risks and prepping for spills or inhalation exposures. The cyano group hints at possible toxicity, and the trifluoromethyl group can escape under severe conditions to form hazardous gases. Handling always means gloves, goggles, and a well-ventilated hood, especially for scale-up. Disposal can’t go in standard waste; proper neutralization and hazardous waste handling protect not just the lab but the wider environment. I’ve known colleagues who underestimated volatility, only to face surprise headaches or skin irritations.
Pharmaceutical development leans heavily on functionalized aromatics for drug discovery and optimization. Medicinal chemists hunt for molecules that last in the body and interact precisely with biological targets, which makes trifluoromethyl-substituted aromatics like this one valuable for both library creation and downstream SAR studies. The electronics industry has looked to such compounds to tweak dielectric properties or introduce new motifs in specialty materials. Agrochemical labs chase these molecules for potential weed and pest control agents, betting on their metabolic stability and unique binding profiles. Even polymer scientists jump in, using similar substituted benzonitriles to impart new characteristics to advanced plastics.
The momentum builds in both academic and industrial labs, where ongoing research finds inventive uses for fluorinated benzonitriles. Scientists zero in on late-stage functionalization, C-H activation, and the tricky business of handling strong electron-withdrawing groups. A review of patent databases shows a steady uptick in new claims involving this scaffold, sometimes as part of kinase inhibitors, other times as a means to strengthen photostability in material coatings. The research community feeds off the challenge, using this compound as part of broader efforts to expand the chemical toolbox for crafting tomorrow’s medicines and materials.
Chemists and toxicologists know better than to trust a cyano group blindly, even in a fairly stable setting. Although specific chronic toxicity data for this molecule sits behind paywalls or in unpublished industry reports, general principles draw from related nitriles and trifluoromethyl aromatics: inhalation or ingestion can provoke central nervous system symptoms, and prolonged skin exposure raises issues ranging from dermatitis to systemic poisoning. Risk assessments cite animal studies or analog data, so regulatory filings generally err on the side of caution. Lab workers already know not to downplay the risk—years of habit mean spotting mislabeling or careless handling.
Looking ahead, interest in 3-Methoxy-5-Trifluoromethylbenzonitrile should keep rising as tailored molecules command more attention in both drug discovery and material science. As synthetic methods become greener and more selective, the accessibility of such complex aromatics should improve, making it easier for labs with modest budgets to run big ideas quickly. Emerging fields like chemical biology and diagnostics also have eyes on these motifs, betting that each new reaction or modification opens an avenue that didn’t exist before. With talent moving between academia and industry and fresh collaborations forming, applications that seemed unreachable just a decade ago now inch ever closer to reality.
As someone who's spent years around lab benches and dusty supply rooms, one thing's clear: purity isn’t just a number on a label. In chemical manufacturing, especially with compounds like 3-Methoxy-5-Trifluoromethylbenzonitrile, a shift in purity can upend months of research or lead to waste on an industrial scale. For labs, buying a bottle means choosing between batches where 97% or 99% can make or break a reaction’s outcome.
It's easy to brush over purity as just another QC metric. No one celebrates getting 99% instead of 97%, but the difference sits deep in the work. Any contamination in a chemical can spark side reactions. Imagine placing an Amazon order for a phone and getting a slightly chipped screen every time—not a disaster, but it wears you down after enough repeats. In chemical terms, these tiny cracks in the supply chain mean lost yield, extra cleanup, and sometimes, complete failure in making what you want.
Drug synthesis has tight margins for error. If a researcher plans a reaction step using 3-Methoxy-5-Trifluoromethylbenzonitrile at 95% purity, even a small impurity could build up in later steps. That residue may show up during analysis or ruin an entire product batch. For electronics, where organic compounds direct the charge flow on tiny chips, nobody wants stray chemicals acting up and ruining a wafer worth thousands of dollars.
Manufacturers push for higher purity using chromatography, crystallization, or distillation, each one scraping away unwanted leftovers from the last run. Costs go up as you chase those last few fractions of a percent. Every added purification step means more labor, solvents, and disposal headaches. In my own experience, arguing for an extra point of purity in budget reviews always sparks debate—everyone wants better results, but nobody wants the bill.
Checking chemical supplier catalogs, most labs find options hovering between 97% and 99%. It’s rare to see 3-Methoxy-5-Trifluoromethylbenzonitrile sold at lower guidelines because customers can’t afford mixed-in unknowns. Sometimes the lot arrives with a certificate of analysis showing an HPLC reading or NMR scan. Scientists glance at the graph, verify that nothing odd sneaks in above 0.5%, and decide to go forward. This isn't a luxury; it’s a defense against wasted weeks of work.
Beyond the numbers, the headache comes from lack of clear information. Suppliers don’t always spell out what that last 2% contains. I’ve worked cases where trace metals or old solvent residues cause confusion. Labs spend valuable hours hunting down contaminants. Open reporting helps—a clear breakdown of all impurities offers scientists a fighting chance. Plus, industry regulations could step in; if authorities set minimum information standards, buyers and sellers would stop playing guessing games.
Stronger communication between suppliers and end-users smooths out many bumps. Sharing batch-specific certificates, better documentation, and even tracing back the raw material sources can reveal whether the chemical stands up to its stated purity. Switching suppliers sometimes solves the problem, though for rare compounds like this, options can get thin. In team meetings, agreeing on acceptable thresholds for purity and side contaminants makes future troubleshooting easier. In my view, respecting those small percent differences saves time, money, and sanity in the long run.
Anyone who has worked in a chemical lab knows storage mistakes can mean headaches—or worse. It doesn’t take a PhD to realize not every powder or liquid can huddle together on the same shelf. I’ve seen enough charred cardboard or swollen reagent bottles to vouch for that.
3-Methoxy-5-Trifluoromethylbenzonitrile, for all its niche applications in pharmaceutical research or materials science, follows some straightforward routines but shouldn’t be shrugged off as “just another bottle.” This compound carries some unique traits: a benzonitrile backbone, a methoxy group, and a cluster of fluorines—each brings something to the handling table.
Heat and moisture change chemicals, sometimes fast and sometimes slow, but seldom for the better. I used to get lazy and ignore humidity warnings, only to discover clumped-up powders and weird color changes. Experience fixed that. For 3-Methoxy-5-Trifluoromethylbenzonitrile, room temperature storage feels safest, but this means under 25°C—excess heat can set off unexpected reactions or slow decomposition that escapes notice until a crucial experiment flops or, worse, puts someone in harm’s way.
Humidity plays its tricks too. This compound doesn’t crave water, but ambient dampness can surely nudge it along towards hydrolysis, especially with open air or cracked stoppers. The best place involves solid shelving in a low-humidity storeroom—think just above freezing winter air, never tropical. Desiccators or moisture-absorbing jars save trouble in the long run, and I’ve found investing in a cheap hygrometer prevents surprises.
Light does funny things to aromatic compounds. In labs, windows seem harmless, but direct sunlight on a chemical can jumpstart all sorts of reactions, especially in compounds with energetic groups like trifluoromethyl. Containers should be brown glass or stored away from any kind of UV lamp. A cardboard box in a cabinet works better than leaving something out for “quick access.”
Air contains more than just moisture. Oxygen finds its way everywhere, especially given enough time. A good seal matters, both for the health of the chemical and for everyone working in the lab. Once, a leaky cap on a similar aromatic nitrile left an odd and biting smell in the storeroom; half the bench workers spent the afternoon with watery eyes. Reagent bottles need airtight caps—no exceptions.
You’d be shocked at how many misplaced or faded labels I’ve run across, especially when older chemicals are in play. I write dates and the full name every time, with hazard symbols where required. It’s easier than an emergency call after a mix-up.
Storing 3-Methoxy-5-Trifluoromethylbenzonitrile means following the Safety Data Sheet, but it also means asking around—talking to a safety manager, checking fire codes, looking up local hazardous chemical rules. An extra set of eyes and a peer check have saved me once or twice when I almost missed something crucial about storage or shelf lives.
Acids and bases belong far away from this compound. It’s better to group it with similar organics—no oxidizers, no reducers, no aggressive cleaning chemicals within reach. A spill kit nearby makes sense, not because you expect trouble, but because you want to be ready if someone else cuts corners.
So whether for new projects or just routine lab work, treat 3-Methoxy-5-Trifluoromethylbenzonitrile with respect—from shelf to label to handling. Experience in the lab proves that careful preparation now means less risk and better science later.
Chemists keep their eyes out for small, clever molecules like 3-Methoxy-5-Trifluoromethylbenzonitrile. It’s not the household name you’ll find in cleaning products or over-the-counter pills, but in the quiet corners of labs, it gets plenty of attention. Think of it like a smart switch that changes the course of bigger, more valuable chemical processes. Its structure—built around a benzene ring, dressed up with nitrile, methoxy, and trifluoromethyl flavors—makes it special. Each of those groups means it behaves differently from other similar molecules. In pharmaceutical chemistry, that difference can lead to new treatments and products.
Pharmaceuticals build new molecules from scratch by patching together different chemical ‘bricks’. This one acts as a brick with three distinct features. Its trifluoromethyl group does more than add weight. It locks in metabolic stability, keeps unwanted breakdown in the body to a minimum, and can change how a drug behaves inside you. The methoxy group tweaks a molecule’s shape and stickiness. The nitrile ends up answering for reactivity—a good spot for other chemical pieces to attach.
A few years back, I helped screen molecules for antipsychotic drug development. We borrowed aromatic nitriles a lot like this compound. The trifluoromethyl group in our leads made the compounds last longer in the bloodstream. Drug companies often use these features to design heart medicines, anti-cancer agents, and compounds for mental health—each step shaped by the functional groups baked into the original molecule.
Fields and farms owe plenty to subtle chemistry tweaks. Crop protection scientists blend complicated molecules to keep yields up and pests down. Fluorinated aromatics, like 3-Methoxy-5-Trifluoromethylbenzonitrile, offer a toolkit for building new herbicides and insecticides. The fluorine content doesn’t just protect crops—it fends off light, water, and microbes, giving the protections staying power.
Some recent patents point out the value of aromatic nitriles in weed killers. The trifluoromethyl branch improves how active ingredients drift through plant membranes, raising their effectiveness where older chemicals stumble. Adding methoxy and nitrile to the mix creates a backbone for refining everything, from how a molecule flows in soil to how it blocks weed enzymes.
People working in fine-chemical and material science labs see similar value. I once met a polymer chemist who used a relative of this compound for making specialty coatings. Those fluorinated arms helped craft smoother, tougher surfaces for industrial and consumer goods. In electronics, researchers hunt for new organic fluorescent materials. Every functional group in this molecule shapes how it interacts with metals, glass, and other organic substances.
It’s easy to overlook the impact of each chemical tweak until you see the finished product—more stable coatings, more efficient screens, sharper diagnostic tools. Small molecules like this one silently boost the performance in a thousand ways, even if most of us never know their names.
Lab scale to mass production is never a simple jump. Fluorine chemistry has a bad habit of being tough to handle—expensive, sometimes unfriendly to factory workers, and not always environmental. There’s a real need for greener, safer ways to make and use these molecules. I’ve watched scientists try less-hazardous reagents and biocatalysis, aiming to cut down on waste and cost. It pays off over time: price drops, safety improves, and the overall environmental mark shrinks.
Looking ahead, the next big win comes from cutting complexity—finding simpler, cleaner syntheses while holding on to the chemical benefits. From what I’ve seen, every new trick that gets 3-Methoxy-5-Trifluoromethylbenzonitrile into the mix more cheaply and cleanly boosts whole industries, quietly nudging medicine, agriculture, and materials to better results.
Ask anyone who's spent time working with chemicals, and they’ll quickly mention the comfort that comes from having a Safety Data Sheet (SDS) at arm’s reach. SDS documents don’t just sit forgotten in a ring-binder — real people rely on them every day. If you look up 3-Methoxy-5-Trifluoromethylbenzonitrile, you'll find that plenty of chemical suppliers put some version of the SDS online. A quick search brings up sheets from major distributors, listing details like hazard identification, first aid measures, fire-fighting steps, and accidental release tips. These aren’t just boxes to tick for compliance. They guide people like chemists and lab techs who mix, measure, and accidentally spill substances like this one.
The catch: not every supplier’s SDS carries the same depth or clarity. Some stick with vague warnings—watch out for skin and eye contact, store sensibly, dispose according to local rules. Anyone who’s sorted through multiple sheets knows this unevenness. Maybe one sheet sketches out what to do if the liquid splashes, but skips over what to expect if you breathe in traces for a month. Sometimes, key toxicology data falls short or isn’t tailored to the actual uses people encounter in research or industry.
I’ve worked on projects where new substances showed up with patchy documentation. That’s a real hazard. You’d have a reaction underway, spot an unexpected vapor, and suddenly realize you’re playing detective. Browsing through the supplier’s website at two in the morning doesn’t cut it for reassurance or safety when the consequences land on your skin or in your lungs.
Getting a copy of an SDS for 3-Methoxy-5-Trifluoromethylbenzonitrile shouldn’t feel like a scavenger hunt. Big suppliers like Sigma-Aldrich generally share well-structured sheets for download, but smaller or overseas outfits sometimes lag behind there. The law does say a supplier must provide a sheet; enforcement varies, and smaller players may drag their feet or use vague translations.
Without reliable data, people turn to lab networks, sharing PDFs over email or forums. That’s a band-aid, not a fix. If the SDS is old or only partly translated, risks multiply fast. Once, I received an SDS written mostly in Mandarin, and my high school grasp of the language turned a hazardous waste spill from a stress test to a panic attack. Others in the lab chipped in translations over coffee, but at that moment, good data felt like borrowed luck, not a guarantee.
Instead of shuffling partial copies and incomplete translations, the field could use open, verifiable SDS databases. If you order a chemical, one QR code on the bottle should deliver you a fresh sheet, updated for regulation and language right away. Governments and chemical associations have the muscle to roll out unified platforms—backed by fines against companies who lag behind.
In the meantime, lab managers and scientists can lead by example. Share well-checked SDS files in a central folder. Push suppliers for updates, and flag ambiguous sheets with a short report directly to regulatory bodies. Even a little collective effort between coworkers or across research labs can lower risk and save a few panicked moments under the fluorescent glare of the fume hood.
Every bottle in the lab comes with a jumble of letters and numbers, and those who spend a lot of time around chemicals know how much a formula can tell you about a substance’s story. For 3-Methoxy-5-Trifluoromethylbenzonitrile, the label reads C9H6F3NO. Its molecular weight comes out to 201.15 g/mol. I can’t count how many times a simple formula answered half a day’s worth of questions before any experiments even started.
Anyone who’s handled this aromatic compound recognizes how those three fluorines make a difference. A trifluoromethyl group stands out, both in the way it pulls electrons and in the way it keeps the whole molecule resistant to breaking down. Out in the wild, that means a compound more stable and less eager to join in with whatever’s around. The methoxy group provides another layer of personality, changing how the substance dissolves and reacts. I remember the first time I measured its solubility—I had to look twice when it dissolved better in organic solvents than I expected. Tried the same thing in water and barely got a trace.
Watch any bench chemist in action and you’ll notice they always check the formula before reaching for a new reagent. This isn't just about mixing the right proportions. In the real world, the structure points to how a molecule might behave down the line. The trifluoromethyl group? It’s more than a set of three Fs on paper—it changes acidity, boiling point, even how the compound travels through the human body. Many new pharmaceuticals use groups like these for just that reason. Each atom in C9H6F3NO counts, since one slip means a failed reaction or missed batch deadline.
Scaling up from milligrams to kilograms looks simple by the numbers, but anyone who’s scaled up reactions knows how quickly those numbers start to work against you. Strong electron-withdrawing groups make molecules tougher to shift around without specialized materials, and sometimes even months-old glassware can spoil a reaction. Then comes the waste: fluorinated byproducts rarely behave. Each step in production needs clever thinking and a willingness to test new routes, which eats up both time and money. I’ve seen teams run out of patience as they cycle through catalyst after catalyst, tweaking solvent ratios for that elusive extra 5% yield.
Smart labs don’t stop at the formula. They bring in analytical chemists, process engineers, and anyone who can hunt for ways to stretch those materials further. Some newer methods swap harsh reagents for milder ones or try out continuous flow setups that allow much tighter control. Recycling solvents and carefully capturing any wasted fluorine offer some relief for both the environment and the budget sheets. Tracking every step, every variable, and every atom is how some outfits keep their process clean and their material costs down.
Reading a label like “3-Methoxy-5-Trifluoromethylbenzonitrile” might feel routine, but anyone who’s actually worked with it knows how much story lives inside that formula. Every part of the process, from molecular formula to cleaner waste streams, needs curiosity matched with experience. That’s how you really turn a handful of letters and numbers into useful science.
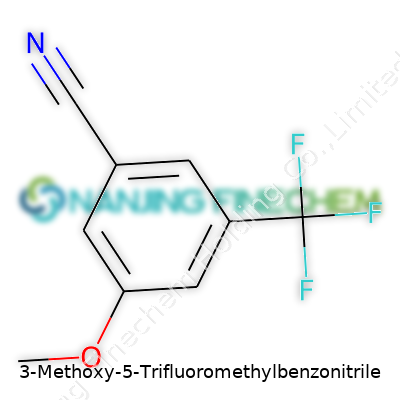
| Names | |
| Preferred IUPAC name | 3-methoxy-5-(trifluoromethyl)benzonitrile |
| Other names |
3-Cyano-5-(trifluoromethyl)anisole 5-(Trifluoromethyl)-3-methoxybenzonitrile |
| Pronunciation | /ˈθriː mɛˈθɒk.si faɪv traɪˌfluːrəˈmɛθɪl bɛnˈzoʊ.nɪˌtraɪl/ |
| Identifiers | |
| CAS Number | 147149-97-1 |
| 3D model (JSmol) | `3D model (JSmol)` string for **3-Methoxy-5-Trifluoromethylbenzonitrile**: ``` CC1=CC(OC)=CC(C(F)(F)F)=C1C#N ``` *(This is actually its SMILES string; JSmol can load this directly as a structure.)* |
| Beilstein Reference | 2086750 |
| ChEBI | CHEBI:162156 |
| ChEMBL | CHEMBL4261067 |
| ChemSpider | 172320 |
| DrugBank | DB08341 |
| ECHA InfoCard | 100.149.145 |
| Gmelin Reference | 10922009 |
| KEGG | C18736 |
| MeSH | D017197 |
| PubChem CID | 153754 |
| RTECS number | GE1228000 |
| UNII | 8HT1G7A30D |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID2057577 |
| Properties | |
| Chemical formula | C9H6F3NO |
| Molar mass | 183.14 g/mol |
| Appearance | White to light yellow solid |
| Odor | No characteristic odor |
| Density | 1.29 g/cm3 |
| Solubility in water | Insoluble |
| log P | 3.2 |
| Vapor pressure | 0.02 mmHg at 25 °C |
| Acidity (pKa) | pKa ≈ 25 (for the most acidic hydrogen, typically on aromatic rings like this) |
| Basicity (pKb) | Product does not contain a basic center |
| Magnetic susceptibility (χ) | -62.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.514 |
| Viscosity | 1.086 mPa·s |
| Dipole moment | 3.59 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -183.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1240.8 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| Flash point | Flash point: 112.7 °C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | SN2265000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Ambient temperatures |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 3-Methoxy-5-Trifluoromethylbenzonitrile. |
| Related compounds | |
| Related compounds |
3-Methoxybenzonitrile 3-Trifluoromethylbenzonitrile 5-Methoxy-3-trifluoromethylbenzaldehyde 5-Trifluoromethyl-2-methoxybenzonitrile 4-Methoxy-3-trifluoromethylbenzonitrile |