Chemical breakthroughs often come from decades of trial, error, curiosity, and the stubbornness of scientists who want to push molecules towards something useful. 3-Amino-2-nitrobenzonitrile didn’t arrive on the chemical stage overnight. Its roots trace through the blossoming years of aniline derivatives in the middle 20th century, where organic synthesis sprouted into an industry changing everything from fabrics to medicines. Researchers found that tweaking benzene rings by applying nitro, amino, and cyano groups opened doors for dyes, pigments, and eventually pharmaceutical starting points. German and American labs appeared in patents and journal footnotes, reporting routes that yielded this chemical as a handy intermediate. Demand came from the growth of fine chemicals and pharmaceutical building blocks, making this molecule more than a footnote. Today, its preparation reflects the old spirit of tinkering, refined by demands for purity, yield, and safety.
3-Amino-2-nitrobenzonitrile stands out among aromatic intermediates thanks to its trio of reactive spots: an amino group, a nitro group, and a nitrile. This combination builds complexity without making the molecule unwieldy, marking it as an ideal candidate when labs want to build up molecular frameworks. Commercial products show off a pale yellow to tan powder, reflecting the nitro group’s legacy as a chromophore. Suppliers pack it in secure containers, mindful of how trace moisture can affect quality or reactivity. The molecule pops up in catalogs for pharmaceutical, agrochemical, and pigment industries—and its demand signals changing trends in specialty organic synthesis worldwide.
3-Amino-2-nitrobenzonitrile, C7H5N3O2 by formula, weighs in at a modest 163.13 g/mol. Its crystalline powder form lets it travel safely between bench and process plant, resisting caking with proper storage outside humid environments. It melts around 151–154°C, reassuring chemists it can stand up to many standard reaction conditions without falling apart. The molecule hardly budges in water—it refuses to dissolve much thanks to the aromatic backbone and nitrile—but it dissolves more easily in organic solvents like ethanol, DMSO, and acetone. That lets researchers play with both polar and non-polar chemistries. Its dual electron-donating and withdrawing groups make for unpredictable reactivity, perfect for driving transformations but demanding care in reaction setup.
Labs and suppliers mark purity benchmarks clearly, often above 98%, rejecting batches with too much trace water or unrelated aromatic contaminants. Certificates of analysis don’t just satisfy regulators—they protect each experiment down the line, as impurities can invite surprise side reactions. Labels show off CAS Number 29685-98-7, along with proper UN codes, handling instructions, and hazard statements. Safety data sheets accompany shipments, spelling out the risks from dust inhalation, skin contact, and long-term storage. Weight, lot number, and storage temperature requirements get prominently displayed, ideal for anyone tracking batch provenance or planning scaled reactions.
The most reliable path to 3-amino-2-nitrobenzonitrile threads through classic aromatic chemistry. One popular approach kicks off by nitrating 3-aminobenzonitrile under controlled conditions—fuming nitric acid and sulfuric acid yield the desired nitro group at the ortho position. Handling the exotherms here separates seasoned chemists from novices, as runaway reactions linger in the memories of any lab worker who’s seen too energetic a nitration. Alternatively, some researchers start from 2-nitrobenzonitrile and use directed amination techniques, leveraging modern transition metal catalysis. Each route brings tradeoffs between cost, yield, scalability, and safety—plants optimize towards whichever offer reproducibility without ballooning hazards or waste streams. Lots of labs favor careful workup steps like recrystallization to ensure high purity, as even small traces of unreacted starting material can complicate downstream chemistry.
Versatility sits at the core of 3-amino-2-nitrobenzonitrile’s appeal. The amino group welcomes acylation, sulfonation, and diazotization, unlocking diazo intermediates important in dye chemistry. The nitro group invites reduction, generating phenylenediamine structures essential for pharmaceutical and agrochemical scaffolds. That nitrile—the most stubborn of the three—brings hydrolysis or condensation options for those willing to apply enough heat or basic/acidic conditions. Building block status means the molecule can leapfrog to bigger, more complex heterocycles wiith just a few clever transformations. Researchers exploit those multiple functional handles when designing routes to compounds that were, only a generation ago, considered out of reach or too expensive for routine synthesis.
Products carry more than one name through catalogs, research papers, and shipping manifests. 3-Amino-2-nitrobenzonitrile, 2-nitro-3-aminobenzonitrile, and 3-Cyano-2-nitroaniline all point to the same molecule. Some suppliers keep things simple and use abbreviations like ANBN. For literature searches, it helps to check all the possible name variants—missing a synonym can mean missing key information about new applications, side reactions, or hazards. Product branding sometimes highlights purity or intended use, but the IUPAC nomenclature still rules for regulatory, patent, and safety tracking.
Make no mistake: 3-amino-2-nitrobenzonitrile, like its nitroaromatic relatives, brings safety considerations. Dust or prolonged contact can irritate skin and mucous membranes, and poorly ventilated spaces risk exposure to potentially toxic fumes during reactions or heating. Personal experience lines up with the warnings—full protective gear and careful bench discipline never get old. Some users keep procedures written up as checklists, minimizing the room for shortcuts. Storage away from direct heat and incompatible reagents (especially oxidizing agents and strong acids) ranks as a must, preserving batch quality and reducing accident risk. Local disposal rules conquer convenience here; contaminated glassware and residues travel towards hazardous waste streams instead of regular garbage. Responding to spills means evacuating non-essential workers and using inert sorbents, not just paper towels.
The real reach of this molecule shows up in the products that quietly fill everyday life. 3-Amino-2-nitrobenzonitrile forms the backbone of many dyes and pigments that appear in everything from textiles to printer inks, leveraging its rapid reactivity and the stability of its aromatic ring. Pharmaceutical companies treat it as gold when piecing together heterocyclic compounds with bioactive potential—think anti-inflammatory or anti-cancer leads. Agrochemical development leans on its reactivity to craft novel herbicides or insecticides, answering the call for more targeted crop protection. Research labs see it as a jumping-off point for exploring new classes of functional materials, especially when searching for molecules with non-linear optical or electronic properties.
More than ever, chemists treat building blocks like 3-amino-2-nitrobenzonitrile as puzzle pieces. The molecule’s functional diversity lets researchers test out new catalysts, synthetic routes, and reaction conditions. Catalysis research thrives on its willingness to react under mild settings, giving green chemistry approaches a real boost. Medicinal chemistry teams harness it to assay hundreds of novel compounds, chasing after better absorption, metabolic stability, or selectivity. Real-time analytic techniques like NMR, LC-MS, and GC now reveal reaction progress, pushing boundaries for yield and selectivity in the hunt for sustainable manufacturing. Institutes and commercial R&D groups chase cost-effective, safer, and cleaner prep methods—this drive carries the field forward, raising product quality across the industry.
With great reactivity comes a burden: toxicity can become an unwanted side effect. Studies on 3-amino-2-nitrobenzonitrile—like those on many nitroanilines—deliver important warnings. Acute exposure can irritate skin and lungs, and some breakdown products may trouble aquatic ecosystems if released untreated. More data rolls in every year looking at mutagenicity, bioaccumulation, and effects on metabolizing enzymes in mammalian systems. Researchers keep exploring structural modification paths to keep the best functional benefits while dialing down environmental and worker risks. Regulatory agencies track animal studies and workplace exposure limits, shaping best practices for handling, protective equipment, and waste disposal.
Today’s progress doesn’t mean the journey ends here. 3-Amino-2-nitrobenzonitrile will end up at the starting line for new generations of drugs and smart materials. As synthetic chemistry keeps shedding inefficient or polluting steps, demand grows for intermediates that deliver flexibility and value. This molecule stands to benefit as manufacturers upgrade processes to greener, safer technology. Regulatory trends and customer scrutiny keep pushing for even purer batches and tighter documentation, and AI-driven molecular design starts to tap its untapped potential in lead compound discovery. Whether it lands in a specialty dye, a field-ready crop protector, or a life-changing pharmaceutical, its role in shaping tomorrow’s chemistry looks ready to grow even larger.
Chemistry has a way of making people’s eyes glaze over, especially once the talk shifts to things like nitro groups and benzonitrile rings. But there’s something honest about breaking molecules down to their bare basics. 3-Amino-2-Nitrobenzonitrile stands as a perfect example of how powerful and revealing chemical formulas can be, even though, on the surface, it seems like just another mouthful straight out of an organic chemistry text.
The formula for 3-Amino-2-Nitrobenzonitrile is C7H5N3O2. This means you have seven carbons, five hydrogens, three nitrogens, and two oxygens, all coming together in a specific dance that gives the compound its personality. Each atom comes with its own history, its own reason for being there. This isn’t just some chemical trivia, either; knowing this formula means understanding what you’re really working with, whether you’re in a high school classroom or a pharmaceutical lab.
Having the right formula doesn’t mean someone is showing off. It’s the kind of thing that can save hours—maybe days—of work in the lab. If a researcher mixes up a compound’s structure, whole batches of a product might turn out wrong. Long ago in my undergraduate days, I saw how one simple misreading of a molecular formula cost an entire week’s worth of research. Glassware stacked up and reactions failed all over a detail that could’ve been double-checked in less than a minute.
Each group on the molecule tells a story. Take the nitro group, hung off the benzene ring: its presence shapes how the molecule reacts, how stable it proves under heat, whether it explodes or fizzles quietly in a test tube. The amino group brings its own flavor, often making the molecule more reactive, pairing and partnering with acids or other chemicals nearby. The nitrile, attached as –CN, often gives the molecule a sharp odor and firms up its place in the world of intermediates for pharmaceuticals and dyes.
Correct identification of chemicals, right down to knowing simple things like C7H5N3O2 for 3-Amino-2-Nitrobenzonitrile, helps tackle bigger issues. Labs work with all sorts of unknowns. Headlines fill up with stories of chemists or students getting hurt or sick after handling mislabeled bottles. Formulas cut through the jargon, creating a universal code. They keep things from going haywire when people from different backgrounds use the same materials.
Out in the real world, companies rely on these formulas to plan for costs, shipping, waste treatment, and safety protocols. The smallest number or a misplaced letter has busted multi-million-dollar deals, delayed shipments, and shut down production lines. All because someone overlooked the value tucked away inside one molecular formula.
The solution comes down to better education, clearer labeling, and a respect for the facts. Databases, smarter tracking software, and visual systems in storage rooms help. But nothing beats developing the habit of questioning and double-checking. It’s not just about avoiding a headache or chasing some grade. For those who spend their days elbow-deep in chemicals, each correct formula signals a little less risk—a better shot at discoveries that might change the world for the better.
Some chemicals don’t get along well with sloppy storage. 3-Amino-2-Nitrobenzonitrile brings a mix of chemical groups to the table, and that makes it a challenge for careless handling. The nitro group has a well-earned reputation for sensitivity, and the nitrile, while a bit more stable, still isn’t something you want absorbing moisture or sitting in the sun. If you crack open a bottle in a steamy summer garage, you’re making life harder for yourself later on.
Nobody keeps this stuff next to the radiator or above the stove. A cool, stable temperature does more than just keep the material happy—it helps you avoid unnecessary risk. Warmth speeds up reactions. That's chemistry 101. A shelf in a room with climate control, something like 20-25 °C, gives more peace of mind. I’ve worked in labs where a jump in temperature goes unnoticed until the bottle gets crusty and yields take a dive.
Small things cause big problems. Once, after a month in a slightly loose jar, one sample drew in just enough moisture to clump and clog equipment. 3-Amino-2-Nitrobenzonitrile has both a nitro group and nitrile—structures that don’t shrug off water. Desiccators and screw-top containers aren’t just “nice to have” here. Use tight lids, check the seals, and stash a good desiccant inside your cabinet. Chemicals become liabilities fast when humidity creeps in.
Leave this pale yellow crystal on a sunny windowsill and you’ve bought yourself a possible headache. Light speeds up chemical changes in many aromatic compounds, and nitro groups don’t appreciate it either. In my time working in academic stockrooms, shielding powders from light ended up being the line between a clean result and a pile of mystery goo. Amber vials or cabinets without a stray shaft of sunlight go a long way.
Mix-ups rank right near the top of avoidable problems. In hectic environments, open shelving turns into a mess fast. This compound should have its own clearly marked space, far from incompatible reagents—especially stuff that’s highly reducing or oxidizing. I remember a single misstep mixing containers leading to an unpleasant clean-up session and a stern safety meeting the next day.
Isolating it isn’t just tidy; it’s about reducing the risk of accidental reactions. Gloves, goggles, and a plan for spill response are part of any basic routine. Accidents feed off distraction. Paying attention to labeling, sealing, and organization isn’t just a lesson from regulations; it’s something hammered in after a spill or unexpected reaction.
Trust in the usual habits—sturdy containers, clear labels, desiccants, climate control, and minimum light. These combine into the backbone of good storage. It doesn’t take a fancy facility or a huge budget, just attention to detail and a bit of experience. The goal isn’t to chase perfection, just to dodge preventable mistakes that cost time, money, and health.
It’s one thing to read spec sheets from chemical suppliers, but reality tells another story. Too often, I’ve opened samples of 3-Amino-2-Nitrobenzonitrile and found the purity just hovering around 97%. Sometimes the label promises higher, but lab results challenge those claims. Most of what’s offered on the market doesn’t genuinely push past 98%. If you ask for the so-called “high purity” stuff, expect to wait, pay a premium, or both.
Chemistry at scale doesn’t cut corners. If I’m after consistent results, anything short of real analysis-backed 98% purity throws up red flags. Residual solvents, trace organics, or unlisted byproducts from the nitration step complicate reactions downstream. One overlooked impurity eats hours troubleshooting failed syntheses or chasing ghost peaks in a chromatogram.
Years ago, I took on a synthesis project where a mere 1% unknown impurity from a batch of 3-Amino-2-Nitrobenzonitrile completely derailed plans. No datasheet apology softened that blow—just lost time and aggravated colleagues. For research and pharma work, even that extra percentage matters, as regulatory submissions tolerate only so much ambiguity.
Theoretically, most catalogs list the compound as minimum 97% pure. If you dig deeper, you notice that experienced chemists quietly distrust the fine print. Purity grades of 98% or “99% trace metals basis” play more like aspirational marketing than real guarantees. The top pharmaceutical distributors sometimes supply authentic 98% batches, but outside these circles, buyers nail accuracy only by sending out their own HPLC and NMR checks.
In bulk procurement for industrial synthesis, purity often slips because suppliers push volume, not refinement. Recrystallization and carbon treatment help, but suppliers hesitate to invest unless clients demand documentation and pay extra. This creates a gap: small labs or early-stage startups just settle for typical supplier grades, while heavyweight buyers enforce stricter contracts and keep their own QC teams busy.
Low purity comes from tolerating shortcuts in the manufacturing process. Pushing for higher-quality chemicals takes more than just “asking nicely” on a sourcing platform. Labs or facilities after more than 98% purity must press for certificates of analysis with real batch data. I’ve haggled with suppliers until they sent fresh analytical results—no template data, no fluff.
Another solution: develop in-house purification steps. Smaller teams working on niche molecules sometimes end up purifying their own 3-Amino-2-Nitrobenzonitrile with column chromatography, sacrificing yield for chemistry they can trust. Bigger outfits negotiate supplier development agreements, investing in cleaner production lines at the source.
For anyone in R&D, keep a skeptical eye on purity claims and build time for extra analysis into your timelines. Budget for small-scale purification runs alongside fresh procurement. Double-check the data, not only the sticker on the bottle. In my experience, it pays to treat every “98%” number with a dose of caution until you’ve run the spectra yourself and seen clean results. Only then can the work move forward with fewer surprises and headaches.
Every time I walk past a pharmacy or see a new medicine being advertised, I know there’s a complex web of chemicals working behind the scenes. One compound that flies under the radar, but really makes a difference in the development of medicines and specialty products, is 3-Amino-2-Nitrobenzonitrile. The name may sound like something out of science fiction, but this chemical shows up in crucial parts of pharmaceutical and chemical manufacturing.
Pharmaceutical researchers don’t just stumble upon new medicines overnight. They rely on a toolkit of building blocks. 3-Amino-2-Nitrobenzonitrile sticks out because of the way its structure lends itself as a starting point for synthesizing more complicated molecules. Scientists often use it to build compounds that fight infection, treat disease, or help with pain. The presence of both an amino and a nitro group sets this molecule up as a versatile piece in creating biologically active compounds.
In my work with chemistry students, I’ve seen how the nitro and amino groups allow for selective reactions. If a student needs to attach a ring or add some branches to create a new antibiotic precursor, this molecule often pops up in their plans. Many modern drugs, whether they target bacteria or work as anti-inflammatory agents, get their start because of this compound’s unique reactivity.
Most people barely give a second thought to the bold colors on their clothes or the brilliant hues in a new tech gadget. Yet specialty dyes would be nowhere without dependable chemical intermediates. 3-Amino-2-Nitrobenzonitrile serves as a backbone for making pigments that hold up under tough conditions, like sunlight or strong chemicals.
It struck me the first time I visited a textile plant just how many steps go into producing a single shade that won’t fade after a few washes. The nitro group on the molecule allows it to accept new components to shift the color spectrum, while the nitrile group can be tuned for different end uses. Whether manufacturers aim for heat resistance or a certain depth of color, they turn to this intermediate for reliability and performance.
Specialty chemicals play a role in more industries than we might expect. 3-Amino-2-Nitrobenzonitrile does its part in agrochemicals, helping create herbicides and pesticides that protect crops. Its structure helps chemists craft molecules that perform specific tasks in the field, such as breaking down only in certain soils or being absorbed by a targeted pest. Farmers count on these features for both productivity and safety.
Some companies invest in ongoing research to tap new uses. Advances in electronics demand high-performance materials for circuit boards and displays. Research teams piggyback on molecules like this one to find the right combination of stability and reactivity. Because this compound handles chemical reactions under a range of settings, it becomes a go-to material for prototypes that need to be tough yet flexible.
It’s not enough just to use powerful chemicals. I’ve found the real world pushes us to consider safety and practical handling. Improvements in how 3-Amino-2-Nitrobenzonitrile gets produced and stored center on minimizing exposure and making sure workers stay safe. Better packaging, smarter extraction and purification steps, and real investment in sustainable chemistry all make a difference. Focusing on education and protective systems means manufacturing doesn’t just stay productive—it gets safer for everyone connected to the process.
3-Amino-2-Nitrobenzonitrile doesn’t exactly roll off the tongue, but this sort of chemical shows up in labs and factories tucked inside research kits and manufacturing lots. In any workplace where chemicals play a role, from small bench-top setups to larger production lines, people count on safety data sheets. For me, an SDS isn’t paperwork—it’s as critical as having the right gloves or goggles ready to go. It’s about trust, about not scrambling in a panic if someone spills a strange powder or gets a whiff of something unexpected.
A worker opening a bottle of 3-Amino-2-Nitrobenzonitrile for the first time could be exposed to dust or accidental splashes. There’s no guessing if the powder might irritate skin or even something worse. Without an up-to-date SDS, reaching for that bottle turns into guesswork. I’ve seen new lab staff reach for acetone or soap and water, hoping they chose right, worrying if that’s enough. People make safer choices when they’ve read clear instructions about what to do after accidental contact or inhalation. Real injuries happen when guesses replace information.
So is the sheet available? The answer is usually yes, if not always at first glance. Suppliers have to provide SDS documents by law. For me, it never made sense to accept “We don’t have that sheet” from a distributor. Sites like Sigma-Aldrich, TCI, or Alfa Aesar regularly host current documentation. Sometimes you’ll need the specific CAS number (for this one, it’s 3167-49-5) to search effectively. With that detail, a quick search or even a direct request almost always brings up the right file.
Larger chemical companies and university safety portals often keep central directories online, open to the public or at least to staff. Anyone with a lab background has probably poked around in these databases. It’s not just a box-checking exercise. Reviewing an SDS can reveal all sorts of information a simple chemical label leaves out: solubility, reactivity, handling advice, symptoms to watch for, the type of fire extinguisher to use, and instructions for medical staff.
Without the sheet, accidents get more serious, faster. There’s also the legal risk: regulators ask about SDS compliance in inspections. Fines pile up and, even worse, workplaces risk shutdowns. Yet on a more personal level, a missing SDS means a safety training session can turn into a guessing game. I’ve watched new workers get flustered and veterans get frustrated, all because the most basic safety document was missing.
Tackling the situation starts with setting a workplace policy to never open a chemical container before finding its SDS. Making sure all incoming bottles come with the right data sheet helps. Team leaders or managers who keep digital archives, updated with every new shipment, prevent last-minute scrambles. Leaning on digital tools is a must: a scanned PDF in a common folder or a phone app with offline access knocks down barriers. Training also plays a big role—everyone should know how to find and read an SDS and not be intimidated by technical wording.
The big step is remembering that safety isn’t paperwork alone. It rides on every lab worker and manager looking out for their crew. No bottle should hit a bench or shelf without backup information. That’s not overkill—that’s just how real safety sticks.
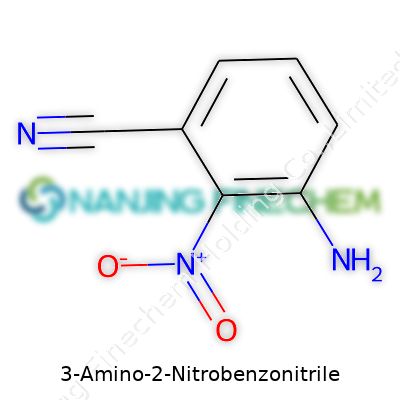
| Names | |
| Preferred IUPAC name | 3-Amino-2-nitrobenzenecarbonitrile |
| Other names |
3-Amino-2-nitrobenzenecarbonitrile 2-Nitro-3-aminobenzonitrile m-Amino-o-nitrobenzonitrile |
| Pronunciation | /ˈθriː-əˈmiːnoʊ-tuː-ˈnaɪtroʊ-ˌbɛnzoʊˈnaɪtraɪl/ |
| Identifiers | |
| CAS Number | 25840-60-4 |
| 3D model (JSmol) | `3DModel:JSmol=CN1C=CC(=C1[N+](=O)[O-])C#N` |
| Beilstein Reference | 2523680 |
| ChEBI | CHEBI:137054 |
| ChEMBL | CHEMBL410026 |
| ChemSpider | 166346 |
| DrugBank | DB08435 |
| ECHA InfoCard | ECHA InfoCard 100.089.646 |
| EC Number | 423-640-6 |
| Gmelin Reference | 811665 |
| KEGG | C14368 |
| MeSH | D052636 |
| PubChem CID | 69460 |
| RTECS number | BY8050000 |
| UNII | V2A6M3M27Z |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C7H5N3O2 |
| Molar mass | 163.13 g/mol |
| Appearance | Yellow to brown solid |
| Odor | Odorless |
| Density | 1.41 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.20 |
| Vapor pressure | 9.89E-6 mmHg at 25°C |
| Acidity (pKa) | pKa = 1.61 |
| Basicity (pKb) | pKb ≈ 9.1 |
| Magnetic susceptibility (χ) | -38.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.727 |
| Dipole moment | 4.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 152.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -650 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P272, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 116°C |
| NIOSH | SW4250000 |
| PEL (Permissible) | Not established |
| Related compounds | |
| Related compounds |
2-Nitrobenzonitrile 3-Amino-2-nitrobenzoic acid 3-Amino-2-nitrobenzamide 3-Nitroaniline 3-Aminobenzonitrile |