Once upon a time, chemistry classrooms introduced basic aromatic compounds. Digging a bit deeper led to derivatives that shaped entire product classes. 3,4-Dichlorobenzonitrile first made waves in the mid-20th century as modern agriculture pressed for reliable weed control. It quickly established its place on industrial production lines in Europe and North America. Over the decades, shifts in environmental policy and market trends pushed chemical manufacturers to refine both process and product. I remember reading early patent documents—recipes required precise temperature control, making plant operators skilled multitaskers. Companies leaned hard on research chemists to optimize each step, from raw kaolin filtration to crystal drying cycles. This molecule didn’t just pop up in chemical handbooks; it emerged from a web of innovation and tough economic reality.
Walk into any specialty chemical supply catalog and you’ll find 3,4-Dichlorobenzonitrile listed under selective herbicides and chemical intermediates. Its transparent, off-white crystalline form doesn’t scream importance, but buyers know demand remains steady. This compound serves as both a direct product for vegetation control and as a scaffold for synthesis of various agrochemicals and specialty dyes. Multinational chemical companies often carry several grades, tailored by purity and crystal size. From field trials in corn and soy fields to pigment production kitchens, this compound’s life stretches far beyond its name.
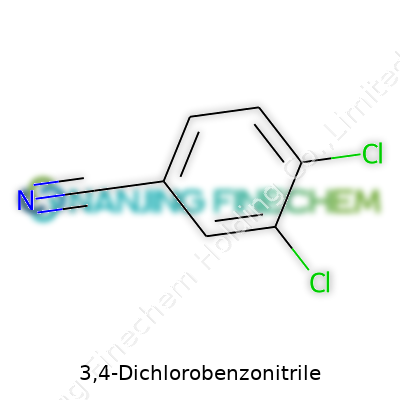
3,4-Dichlorobenzonitrile, formula C7H3Cl2N, shows up as a white or faintly yellow powder. Sharp melting point around 139–140°C and molecular weight just over 172 g/mol make it reliable for calibrated measurements, whether you’re in a university lab or running bulk batches. Solubility checks keep revealing low water compatibility, though it dissolves in acetone and aromatic solvents. Rigorous temperature monitoring matters during storage; thermal decomposition kickstarts above 300°C, releasing harmful fumes. Based on personal encounters at company warehouses, the chemical odor—pungent, slightly sweet—remains a dead giveaway for unsealed containers.
Buy a drum of 3,4-Dichlorobenzonitrile and the technical sheet sticks close. Industrial standards push for a minimum purity above 98%. Packaging usually involves lined fiber drums or HDPE containers to keep moisture away. Each shipment has UN number 2811 and hazard pictograms warning handlers about toxicity. Labeling complies with GHS requirements, including R-phrases for environmental hazard and handling instructions in multiple languages. Over the years, trade shows made me realize label accuracy can make or break an entire shipment at customs, since missing details on reagent label can spell major fines.
Chemical synthesis of 3,4-Dichlorobenzonitrile has stuck to the Sandmeyer pathway for most of its history. Start with 3,4-dichloroaniline and introduce sodium nitrite under chilled, acidic conditions to yield the corresponding diazonium salt. Pour in copper(I) cyanide and the nitrile bond snaps into place. Batch scalability challenges haunted early practitioners, since controlling diazotization temperature often proved tricky. Modern facilities use semi-automated reactors to limit spill risks and push yields over 85%. By-products, mainly salts and unreacted amine, drop out in the aqueous phase, demanding robust waste treatment steps.
In the hands of a skilled synthetic chemist, 3,4-Dichlorobenzonitrile acts as a launchpad for further functionalization. Reduction with LiAlH4 produces the primary amine, a useful intermediate for building pesticides or pharmaceutical molecules. Nucleophilic aromatic substitution gives rise to a string of ether, amine, and alkyl derivatives. Cross-coupling reactions using palladium catalysts extend the molecule’s reach into specialty dye markets and advanced materials. Sitting at a bench, I once watched a colleague create a whole suite of biaryl compounds starting from this chemical, streamlining a process that previously spanned multiple days. Chemical reactivity doesn’t just serve academic curiosity; it drives whole product lines in industrial chemistry.
Chemical catalogs love aliases. 3,4-Dichlorobenzonitrile pops up as Bifenox Nitrile, DCBN, and technical names like 3,4-dichlorobenzenecarbonitrile. US regulatory documents sometimes call it dichlobenil, especially in registration filings with EPA and EU REACH lists. Asian suppliers use variations of the IUPAC name, adding to occasional confusion on import documentation. These alternate names shaped my early ordering mistakes, reminding me that a solid reference table beats guesswork any day.
Anyone handling 3,4-Dichlorobenzonitrile in bulk faces genuine health risks, including respiratory irritation and skin sensitization. Proper PPE makes a difference — gloves, goggles, and particulate masks form the daily armor for technicians. OSHA guidelines limit airborne exposure, and training drills prep workers for accidental spills. Safety data sheets flag the risk of aquatic toxicity, so storage protocols include secondary containment and regular leak checks. I recall site safety audits, inspectors combing storage rooms for even a single granule outside labeled bins. Cleanup plans often spell out neutralizing agents and disposal routes to keep workplace and environment safe.
3,4-Dichlorobenzonitrile’s largest arena remains selective herbicidal action. Applied to non-crop and ornamental sites, this compound suppresses weed growth at the germination stage, reducing labor costs and protecting ticket crops. Besides herbicide uses, pigment and dye industries use this nitrile as a building block for bright, high-performance azo dyes. Specialty manufacturers engineer further transformations for use in photoresists and advanced polymers. I’ve watched technical managers balance application dose precisely, since overuse can trigger regulatory pushback or damage nearby aquatic habitats.
Each year brings improvements through research into process intensification and greener production techniques. Chemists tweak reaction steps to reduce energy demand and limit hazardous by-products. Collaborative industry-academic efforts focus on biocatalytic routes and solvent minimization. I once attended a university-led workshop where a pilot process promised to cut cyanide-based waste in half. Startups aiming at sustainable chemistry keep their eyes on these advances to remain competitive and meet stricter rules on emissions and worker safety.
Animal studies and environmental assays paint a clear picture of 3,4-Dichlorobenzonitrile’s toxicological impact. Acute toxicity remains moderate but amplification occurs with prolonged exposure, especially in aquatic systems. Fish and invertebrates show marked effects at low microgram levels. Regulatory reports cite soil persistence as a moderate concern, pushing for strict rotational use and buffer zones near water sources. Clinical data among exposed workers highlight reversible symptoms like headaches and skin rash rather than systemic organ damage. Public databases like TOXNET helped me corroborate what workplace stories already suggested: continuous vigilance trumps complacency, both for users and the surrounding ecosystem.
Rising demand for crop protection and specialty chemicals keeps pressure on suppliers to balance production scale with responsible stewardship. Markets in Asia-Pacific and Latin America grow fastest, driven by expanding agricultural acreage and tightening weed management rules. Environmental concerns push for new synthesis methods, focusing on waste reduction and renewable starting materials. Industry groups partner with regulatory bodies on developing safer alternatives and time-release formulations. My visits to trade conventions highlighted an emerging consensus: sustainability upgrades win contracts and public trust, not just regulatory approval.
3,4-Dichlorobenzonitrile sounds like the kind of scientific term that makes most folks’ eyes glaze over. But peel back those complicated letters and you'll find a chemical that's pretty common in the agriculture space. Specifically, it goes by the trade name DCBN, and farmers recognize it as the active ingredient in certain selective herbicides. This compound plays a key role in keeping crops clear of pesky intruders—weeds that could steal light, space, and nutrients.
Many people who’ve never had to pull weeds by hand or lost a row of beans to a stubborn invader miss how much of a fight it is to keep fields productive. Yields can plummet fast. Using DCBN, certain growers can target weeds early and spare their crops a losing battle. For example, in onion and garlic fields, DCBN helps farmers knock out weeds right as seedlings push through the dirt. These crops grow slowly at first, and weeds can outcompete them with ease.
People rely on DCBN because it works well on annual grasses and some broadleaf weeds. Its strength is in its selectivity—it damages unwanted plants but spares crops if applied at the right time and in proper doses. That’s only part of the story. After a few years in the business, it becomes clear that too much reliance on a single weed-control solution can backfire.
A problem farmers talk about is resistance. Some weeds learn to shrug off chemicals, and then the old tricks stop working. Glyphosate, another common weed killer, ran into this problem. Now, experts worry that overuse of DCBN could send us down the same path. Keeping weeds in check needs a mix of approaches—rotation, mechanical tools, even going after young weeds with the hoe before they get strong.
We hear a lot about pesticide dangers, and with DCBN, there’s real cause to pay attention. The Environmental Protection Agency requires caution: limited use, buffer zones, and strict safety gear. DCBN can stick around in soil and water, so runoff matters—especially near sensitive streams or homes. Folks working in treated fields have to wear heavy gloves and protective eyewear. I remember the smell lingering on clothes long after application and the warnings to never skip the mask.
Consumers may ask: does any of this wind up on my food? Regulatory watchdogs set limits and test for residues. Yet concerns over long-term health remain—it’s tough to ignore those stories about contaminated groundwater and sick farmworkers. If a safer, effective option showed up, most people I know would jump at the chance to quit using harsh chemicals.
For now, 3,4-Dichlorobenzonitrile is part of how large-scale food production happens. There’s no clear replacement with the same effectiveness, but change is happening. Some farms blend DCBN with natural weed blockers or tweak seeding schedules to give crops a head start over weeds. Others shift toward organic methods and cover crops, even if it means more sweat and risk. New technology—like sensors, weed-zapping robots, or custom seed drills—lets growers spot-treat trouble spots instead of blanketing whole fields. Each season brings another round of trial and error, innovation, and lessons learned the hard way.
If you’ve ever worked in a place with shelves lined by mysterious bottles, you know every new label brings a sense of caution. 3,4-Dichlorobenzonitrile is one of those chemicals that sets off the mental hazard alarms. It’s used in labs and sometimes in making herbicides. The risks aren’t just on some distant safety sheet—anyone working with it comes face to face with how fragile skin, lungs, and eyes can be.
The stuff is toxic. Inhaling its dust irritates airways, and getting it on your skin isn’t any better. Eyes suffer the most from splashes; even gloves can quickly degrade if you choose the wrong kind. I remember back in grad school, a friend spilled just a little bit on his glove, didn’t notice, and ended up with a red, patchy finger for a week. So, chemical safety stops being a bureaucratic exercise and starts looking like just trying to go home healthy.
Respecting this chemical starts with gear. Forgetting goggles or thinking a dust mask does the job can mean pain and regret. Splash goggles, a well-fitted lab coat, and nitrile gloves go a long way. One pair of gloves usually isn’t enough—nitrile over latex, for example, shields better against degradation. Any sign of cracking or fogging means those gloves are done. Respirators help, especially if the lab air isn’t great or processes produce dust. The right filter—one rated for organic vapors—matters.
I’ve lost count of times someone tried to “just open a window” for fume-heavy tasks. That never cuts it. 3,4-Dichlorobenzonitrile shouldn’t get anywhere near your lungs. A chemical fume hood makes a world of difference. It captures dust and keeps vapors out of the room. If the workplace invests in a good hood and makes sure people actually use it, accidents drop.
Don’t just stick the chemical on any spare shelf. Tightly closed, clearly labeled, and far from acids and oxidizers, that’s the benchmark. Once, in an old lab, we found a half-open bottle crammed behind cleaning products—pretty much my nightmare scenario. If you spill, dry material shouldn’t touch bare hands or any regular paper towels. Simple absorbent pads and stiff brushes meant for chemical duty work better. After that, the residue needs approved disposal. Drains and trash bins aren't the place for it.
Reading the SDS counts for little without clear training and reminders. Watching someone handle the substance sloppily as a rookie made me tighten up. Ongoing practice and refreshers go beyond memorizing rules—they embed habits. The labs and factories with the best safety records tend to say, “Let’s walk through this together” instead of, “Here’s a manual—good luck.”
Workers always pick up signals from management. If safety gear runs out or corners get cut to save time, injuries rise. Protecting people starts with management treating supplies and proper training as non-negotiable. Proper disposal bins, eyewash stations, and regular audits aren’t extras. They’re basic signs that people matter. Handling 3,4-Dichlorobenzonitrile safely isn’t about ticking off boxes—it’s about respecting health and waking up the next day with no regrets, fingers and lungs intact.
3,4-Dichlorobenzonitrile, a mouthful by any standard, goes by the formula C7H3Cl2N. Years back, standing in a lab that barely seemed big enough for my ideas, I first encountered little off-white crystals that formed the backbone of this very compound. One whiff too close to a sample and a sharp, chemical tang reminded me often that some things demand respect in the lab.
Open a new bottle, and you’d see a pale, almost white crystalline powder. The granular look almost tricks you into thinking of sugar, but its story runs much deeper. That almost anonymous appearance hides its major role in agricultural and chemical processes.
Hitched a ride to a big farming research operation a few years ago, and I saw firsthand how unwanted plants threaten to take over every patch of green. Many growers trust 3,4-Dichlorobenzonitrile for weed management—not just for show either, because the compound knocks out select weeds and spares the crops. No field ever feels simple, yet one selective tool can tilt the balance toward higher food output. The work it does in keeping invasive species at bay saves countless manual hours and helps feed families more reliably.
In the synthesis world, this compound becomes a stepping stone, joining reactions and forming new molecules used in everything from dyes to specialty products. That pale powder may not seem impressive, but it shows up in plenty of chemical catalogs for a reason. Folks in the lab value consistency and a predictable reaction partner—a steady hand like 3,4-Dichlorobenzonitrile keeps the gears moving. Scientists often lean on its stable form and reactivity, knowing the results won’t jump around and surprise them.
No shortcut comes free. In the wrong hands or without safeguards, 3,4-Dichlorobenzonitrile can pose health risks. Watching a colleague brush off a bit of powder and shrug always made me uneasy; inhalation or skin exposure doesn’t just cause discomfort, it can do real harm. This isn’t paranoia—it’s experience. More than once, I’ve seen the need for clear rules and proper storage. Gloves, eye protection, and plenty of ventilation aren’t optional; they’re the border between routine work and the emergency room. That goes double when storing the compound, since warm or humid spaces can break down the product and release fumes that nobody wants to breathe.
Communities often ask why these chemicals stick around, especially with worries about runoff into water supplies. As an old chemistry mentor quipped, “Solutions drive progress.” Researchers work hard at finding targeted methods or even alternative compounds less likely to disrupt ecosystems. Some growers are testing precision sprayers and timing their applications to limit amounts needed. On the lab front, green chemistry has gained ground, with teams designing similar products that break down more easily. In the daily shuffle of science and farming, small steps count for a lot. Progress only happens when everyone at the table—growers, chemists, consumers—keeps talking and weighing tradeoffs together.
3,4-Dichlorobenzonitrile is no stranger to anyone who's spent time in a lab or a storage room that smells a little too sharp. This compound, used as a herbicide and in chemical synthesis, doesn't just sit on the shelf quietly. Mishandling it leads to chemical leaks and even health issues. That sharp, chlorinated scent signals danger, not something to take lightly.
Anyone who’s opened a drum and caught a strong, irritating whiff knows this chemical means business. It causes skin and eye irritation pretty quickly. Even brief exposure leads to sneezing or coughing. I once saw a coworker break out in a rash after brushing some dust off his gloves — a reminder of what happens without proper storage and handling. The risks become much bigger in warm rooms or places with poor ventilation.
Forget tossing the drum onto any random shelf. 3,4-Dichlorobenzonitrile needs a dry, cool spot far from anything that might spark or create heat. Heat speeds up its breakdown, and, sometimes, that leads to the release of nasty byproducts. Moisture isn’t a friend either. Crystals draw in water, start clumping, and before you know it, the material’s not just hard to use — it’s a hazard. A leak becomes much harder to control when water’s involved.
Stacking chemicals on top of each other saves space, but with active compounds like this, every shortcut invites disaster. Keep oxidizers, strong acids, or alkalis away from the area. A spill here or a cracked bottle there sets off unplanned reactions, some of them toxic or corrosive. I’ve seen two careless suppliers unpack unrelated raw materials in the same corner, only to spend the afternoon mopping a yellow mess after a leaking bottle started reacting with pool chemicals nearby.
Cheap plastic falls apart under pressure. A chemical like 3,4-Dichlorobenzonitrile calls for sturdy, sealed containers — thick-walled polyethylene or metal drums lined with protective coatings. Damaged packaging lets off powder or vapors, which quickly fill up tight storage rooms. Strong seals and clear hazard labels don’t just tick regulatory boxes. They warn newcomers and remind old hands to use gloves and goggles before moving anything.
A stuffy storage closet does nobody any favors. I always preferred working with facilities that invested in steady airflow. A vented storage room clears away accidental vapors fast. On top of that, keeping floors and shelves clean ensures no residues linger, mixing with other chemicals. Regular checks once a week — inspecting seals, cleaning up spilled dust, replacing peeling hazard stickers — keeps everything under control.
Storing chemicals isn’t just about space; it’s about knowledge. People should know the risks and not take shortcuts. Safe handling training means workers don’t have to guess where to find gloves or what a chemical odor means. An eyewash station nearby, along with quick access to spill kits, shows the difference between a near-miss and a long hospital visit in case something goes wrong.
Companies owe it to their staff to maintain decent storage rooms and never tempt fate by overcrowding shelves. Regular reviews, honest risk assessments, and a culture where people speak up instead of cutting corners keep both employees and the environment safe. Everyone remembers the fume-filled afternoons or hard lessons learned. The story is pretty straightforward: Invest the time up front, and the rest falls into place naturally.
3,4-Dichlorobenzonitrile pops up often in the world of industrial chemistry. For many, it comes off as just another compound among thousands, but folks in the business of plant protection and fine chemical synthesis tend to watch its quality closely. The punch it packs in terms of reactivity rests squarely on its purity and grade—something anyone handling or buying it should pay attention to. I remember standing on the production floor at a plant in Gujarat, hearing arguments break out over purity percentage points—turns out, a decimal can make or break downstream results.
The specs go way past a basic “white powder” description. Chemically, it carries a molecular formula of C7H3Cl2N, with a molecular weight around 172.01 g/mol. Most suppliers sell the stuff as an off-white powder or sometimes crystalline solid, which tells experienced hands straight away to check for moisture or caking—two early warning signs of trouble.
As for melting range, the numbers usually settle between 140°C and 145°C. In my own lab days, that melting point gave us a quick test for quality: anything dropping below meant impurities had snuck in or water had been absorbed. Moisture content shouldn’t tip past 0.5%. Hitting that number consistently means someone’s paying attention to packing and storage. Levels above 0.5% show up fast as clumping, which nobody wants, especially those using it in fine chemistry.
This chemical often comes in grades that hover between 97% and 99%. Every percentage point up or down changes the conversation. At 97%, you’re looking at material that’s fine for most synthetic or industrial processes. For work that goes toward pharmaceuticals, 99% or above makes a big difference, as any stray chemical or trace contaminant can mean a failed reaction or a safety risk. I’ve seen batches get rejected by pharma companies for not meeting tight purity limits, something that causes both frustration and extra cost.
Lower-quality grades, around 95%, do turn up—often they wind up in basic herbicide production or industrial sectors where a micro-percentage of impurity won’t affect critical outcomes. Folks in food or API manufacture can never use these grades, and that’s a rule you can take to the bank.
This compound can pick up impurities from starting materials or equipment, such as residual acids or heavy metals. Good suppliers run basic tests for trace iron, lead, or other metals. Any reading for heavy metals above 10 ppm raises red flags—regulations and customer specs make that pretty clear, especially in Europe and North America. Some manufacturers list chloride or sulfate levels in their specs, which helps track down sources of contamination if downstream problems pop up.
If you’re sourcing, don’t just fixate on the top-line purity number. Pay attention to the moisture, ash, and contaminant levels. Ask for a certificate of analysis from any serious supplier and read between the lines. On-site verification with your own lab, if possible, beats taking a spec sheet at face value. I’ve seen buyers rely on paperwork alone, only to discover off-colour or off-smell batches—usually a trace of a skipped process step.
For companies struggling with inconsistent quality, investing in better drying, closed-system handling, and high-quality packaging cuts down moisture pick-up and cross-contamination. On the buyer’s end, storing the chemical in dry, sealed containers and tracking batch numbers keeps surprises at bay. This isn’t just about cost; it’s about knowing what you’re really getting and what risks you’re running, especially if you’re working up the supply chain or handling it in a regulated space.

| Names | |
| Preferred IUPAC name | 3,4-dichlorobenzene-1-carbonitrile |
| Other names |
Benzonitrile, 3,4-dichloro- 3,4-Dichlorobenzonitrile 4-Cyano-1,2-dichlorobenzene 3,4-Dichlorobenzene carbonitrile 4,5-Dichlorobenzonitrile |
| Pronunciation | /ˈθriː,ˈfɔːr daɪˈklɔːr.oʊˌbɛnz.oʊˈnaɪ.trɪl/ |
| Identifiers | |
| CAS Number | 3018-12-0 |
| 3D model (JSmol) | `3d:CC1=CC(=C(C=C1Cl)Cl)C#N` |
| Beilstein Reference | 1207932 |
| ChEBI | CHEBI:34661 |
| ChEMBL | CHEMBL15829 |
| ChemSpider | 11916 |
| DrugBank | DB08220 |
| ECHA InfoCard | 03e3ce13-83e1-466c-aac4-9f5ceb523316 |
| EC Number | 223-498-3 |
| Gmelin Reference | 82144 |
| KEGG | C14333 |
| MeSH | D003999 |
| PubChem CID | 6951 |
| RTECS number | CZ1925000 |
| UNII | VAD260P7ZQ |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C7H3Cl2N |
| Molar mass | 153.01 g/mol |
| Appearance | white crystalline solid |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.9 |
| Vapor pressure | 0.011 mmHg (25°C) |
| Acidity (pKa) | 2.54 |
| Basicity (pKb) | 1.68 |
| Magnetic susceptibility (χ) | -81.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 1.56 mPa·s (20°C) |
| Dipole moment | 2.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -30.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3060 kJ/mol |
| Pharmacology | |
| ATC code | N06AX15 |
| Hazards | |
| Main hazards | Harmful if swallowed. Harmful in contact with skin. Causes skin irritation. Causes serious eye irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P362+P364, P391, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | 111°C |
| Autoignition temperature | 540°C |
| Lethal dose or concentration | LD₅₀ oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): 648 mg/kg (rat, oral) |
| NIOSH | TT8050000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) of 3,4-Dichlorobenzonitrile is "0.5 mg/m³". |
| IDLH (Immediate danger) | Probably 20 ppm |
| Related compounds | |
| Related compounds |
3,4-Dichloroaniline 3,4-Dichlorobenzaldehyde 3,4-Dichlorobenzyl alcohol 3,4-Dichlorotoluene 3,4-Dichlorobenzoic acid Bromoxynil Ioxynil |