Interest in amines popped up fast after chemists discovered simple reactions between alcohols and ammonia back in the 1800s. 2-Butylamine didn’t earn much attention until large-scale organic synthesis ramped up in the 20th century. Production tracked alongside the rise of the petroleum and pharmaceutical industries. Once the Haber-Bosch process made ammonia cheap, the scene was set. Chemical manufacturers soon realized 2-butylamine could open doors to all sorts of agrochemicals, rubbers, and other specialties. Chemists fiddled with conversion routes from olefins and alcohols, sometimes just by trial and error. Industry patents from the 1950s outline applications in pesticide intermediates and stabilizers—old lab journals from that era smell a bit like 2-butylamine today.
Holding a spot as a colorless, flammable liquid, 2-butylamine doesn’t jump out at you visually. Its main draw comes from how the butyl group hooks onto the nitrogen atom, creating a compound that launches into alkylation and acylation reactions. Chemical catalogs show its ability to slip into agricultural chemicals, accelerators for rubber production, corrosion inhibitors, and a list of specialty solvents. Some folks in the academic world also grab it for research into organic synthesis, since the amine lends itself well to tweaking molecular backbones.
Anyone who’s opened a bottle of this amine knows about the sharp, ammonia-like odor that doesn’t quickly fade from nose or clothing. It flashes a boiling point near 77°C, making it volatile enough to demand respect in a warm lab. Density sits around 0.74 g/cm³ and it dissolves pretty freely in water or most organic solvents. This basicity, thanks to the lone nitrogen, determines how it behaves during chemical reactions. It combusts easily and the vapor can irritate skin and eyes, so experience backs up every warning you see on the drum.
2-Butylamine usually gets sold at more than 98% purity, unless custom requirements call for a stabilizer or minor additives. Packaging comes in steel drums with tight seals to cut down the risk of evaporation, and every shipping label flashes the hazard diamond for flammable liquids and toxic materials. Safety Data Sheets list UN2733 for transport regulations. Manufacturers note its CAS number, 109-73-9, and often highlight GC-MS chromatograms to assure customers of product consistency.
Most industrial plants lean into reductive amination of 2-butanone with ammonia, using a suitable hydrogenation catalyst—nickel or platinum standbys do the lifting. The batch set-up usually starts with mixing ammonia and the ketone in a pressurized vessel, heating things up while feeding in hydrogen. After cooling and separation, distillation strips off excess solvents. Some plants opt for direct amination, working from butyl halides with aqueous ammonia, trading off a bit of atom economy for fewer by-products. These methods grew up side by side with improvements in catalyst recovery and recycling, not just to cut costs but to address mounting environmental regulations.
The free amine group gives chemists a wide canvas. 2-butylamine takes on acyl groups in one-pot syntheses. Many use it to build ureas, isocyanates, and sulfonamides—handy for both drug and agrichemical discovery. As a nucleophile, it snaps up alkylation partners, especially in the hands of process chemists chasing new ligands and surfactants. My first taste of this involved a rather smoky alkylation, and I learned immediately how a lack of fume hood discipline can stay with you for a week.
Globally, 2-butylamine also responds to sec-butylamine and 1-methylpropylamine. Catalogs use these names interchangeably, but make sure to watch out for isomer confusion; there’s a reason labels highlight the “sec-” prefix. Some regions tag it under EC number 203-699-2 and FEMA number 3601, depending on market destination. None of these variations change the chemistry itself, only the comfort level for customs paperwork.
Anyone handling 2-butylamine spends time reviewing its hazard profile. Flammable vapors mix with air and form explosive blends, so gear up with grounded equipment and avoid any ignition sources. Standard procedures recommend chemical-resistant gloves, splash goggles, and good local exhaust. The NFPA rates it with a three for health and two for flammability. Absorption through the skin or inhalation can trigger anything from headaches to severe respiratory distress—cold facts, but real concerns for anyone spending hours in a process plant or bench-scale set-up. Spill kits and emergency showers stand within reach, with proper ventilation preventing build-up of dangerous vapor concentrations.
Rubber manufacturers picked up 2-butylamine early for use in accelerators, stepping up vulcanization efficiency and giving car tires longer life. Agricultural companies blend it into intermediates for pesticides designed against a range of weeds and pests. In corrosion inhibition, it gets added to oilfield and boiler treatments where it slows down decay of metal surfaces. I’ve met formulating chemists in water treatment and pharma who keep 2-butylamine on hand for small-batch pathway studies, especially if they want to introduce a branched-chain amine and see how it changes the end product’s behavior. Some literature points to it as a building block for specialty solvents and flotation agents in mining.
Academic teams continue playing with 2-butylamine to seed new ligands and catalysts. Recent papers dive into its use in designing metal-organic frameworks and exploring reactivity trends across homologous amines. Start-ups in the sustainable chemistry sector also look at ways to produce it from renewable feedstocks, using microbial transformation of plant sugars or waste biomass to cut reliance on petrochemicals. Patent filings shoot up every few years when someone discovers a new synthetic route or application, keeping this unassuming amine in the spotlight for industrial innovation.
Toxicology data for 2-butylamine warns about moderate to high acute toxicity. Direct skin exposure or inhalation causes burns, respiratory irritation, and in rare cases, systemic toxicity. Lab animal studies chart LD50 values in the hundreds of milligrams per kilogram, with most incidents stemming from accidental spills or vapor inhalation. Chronic exposure links to CNS depression and some liver effects, so occupational safety groups recommend rigorous monitoring and exposure limits—usually capped at 5 ppm averaged over an eight-hour shift. Monitoring badges help plants catch spikes in vapor concentration fast.
Emerging green chemistry goals keep 2-butylamine relevant. Researchers push for new bio-based synthetic technology, aiming to keep production cost-effective while slashing emissions and energy use. Start-ups scout pilot-scale reactors using engineered biocatalysts, hoping the next breakthrough can add value to agricultural byproducts. Process optimization—getting more product per unit of raw material—remains a constant focus. Any regulatory move reducing solvent toxicity will spark new approaches for handling, storage, and downstream processing. Climate policy and sustainable materials initiatives may boost demand for specialty amines like this over the next decade. Experience tells me that any well-established reagent with a clear functional group and reliable chemistry will hold its ground, provided it adapts with environmental and safety trends.
You walk past a chemical plant or flip through the back of a cleaning product label and run into names you’ve never spoken, like 2-butylamine. Rarely does this chemical ring bells at the dinner table, but its role outside the lab matters more than most people think. Chemistry might look distant, but these building blocks shape products we meet every day.
2-Butylamine pops up on ingredient lists because chemists value its structure — a four-carbon chain with an amine stuck to it. This mix gives it some interesting properties that find their way into a few different corners of industry. Years back, I had a chance to work with specialty chemical suppliers who emphasized molecules like this turn up in more than one place, even if nobody takes notice.
One of the main uses of 2-butylamine happens in the creation of pesticides. Farmers and orchard hands rely on crop protection, and not many realize these active agents often get crafted using little molecules gathered from the shelves of chemical warehouses. 2-Butylamine acts as a starter—what chemists call an intermediate—helping to link together more complex ingredients found in fungicides and herbicides. This allows the final product to stick to leaves better or break down in a safer way once crops come to harvest.
Medicines can look complicated, but look close enough and some repeat players show up. 2-Butylamine often serves as the skeleton that holds bigger, more sophisticated pharmaceutical compounds together. Drug companies use it to help make antihistamines or antidepressants. During production, it helps control reactions, creating smoother paths to more effective pills and capsules. Researchers sometimes point out how tricky it can get to fine-tune medicines, and a well-chosen amine like this one can tip the balance between a breakthrough and a waste of a year’s work.
The tires under a car, the seal around a window, even some household plastic parts—these products all need a finishing touch to reach the right feel, stretch, or resistance to weather. 2-Butylamine lends a hand as a curing agent or accelerator. The tire industry grabbed my attention long ago due to how much rides on formulas people never see. Using the right mix can carve years of extra wear out of tires or save time during production, reducing energy costs and emissions.
Handling chemicals like 2-butylamine asks for respect. Exposure can affect health—nobody needs headaches, irritation, or worse. Companies keep tight controls regarding storage, ventilation, and waste management. I remember a plant manager telling his staff, “Treat every drop like it’s worth its weight in gold,” and he meant in safety as much as expense. Stricter standards bring down accidents, and regular checks along with clear labeling close the gaps where mistakes creep in.
Chemists always look for ways to use less hazardous alternatives. Some manufacturers test greener processes or substitute raw materials when practical. Collaboration between industry, government, and researchers pushes these efforts along. I’ve watched trade shows filled with engineers fixated on safety upgrades that could replace volatile building blocks like 2-butylamine. Replacing just a small fraction of its use would mean cleaner air for neighbors and safer hands for workers.
2-Butylamine may not draw crowds, but it helps bring better yields to farms, medicines to hospitals, and performance to everyday products. The next time you dig into how everyday goods are made, it pays to recognize that small molecules often make the real difference—not just the brand names on the label.
People outside a laboratory probably never hear about 2-Butylamine. To most, it’s just a name scribbled on a bottle that’ll gather dust in the corner of a supply cabinet. In reality, each of those names leads back to a specific identity, and in science, identity starts with a number. For 2-Butylamine, that number is 109-73-9. This is the CAS number—a local language for chemists, regulators, importers, and folks checking up on safety sheets before they order a barrel. It doesn’t just mark a spot on a list. It tells people exactly what they’re working with, clearing up confusion when trade names or synonyms get thrown around.
Years ago, someone in my research group ordered a chemical under its trade name. It looked fine enough until someone noticed the safety data sheet didn’t match the reagent’s structure. The order almost caused a week-long delay and could have led to a waste of several thousand dollars. Backtracking through the paperwork, we found the mistake—no CAS number used, miscommunication bloomed, and money burned.
Now, ordering anything without double-checking that string of numbers feels reckless. Chemical names shift across languages and companies, but that CAS number stays steady. If you need 2-Butylamine’s CAS, you look for 109-73-9, and you’ve got the right compound no matter if you’re in Minnesota, Mumbai, or Mannheim. That’s more than just bureaucracy. It builds trust and consistency into an industry that depends on details.
Working with amines like 2-Butylamine means attention matters. It’s not just about missing a target product, but about avoiding hazards—both in the lab and further down the supply chain. Mix up CAS numbers, and someone could grab something toxic, flammable, or environmentally risky when they need a simple precursor. Accidental swaps don’t just stay in the science world; accidents ripple outward. Freight handlers, environmental officers, and emergency responders downstream need clear and precise information too.
That sort of reliability doesn’t develop by accident. The Chemical Abstracts Service put in the work so every molecule gets a unique registry number. 2-Butylamine got its slot as 109-73-9, and anyone who works with chemicals can lean on that number to speak clearly about what they mean. Schedules, efficiency, and health rely on that clarity.
If there’s a step to fix the confusion, it starts with keeping CAS numbers front and center—on all documents, in emails, on orders. Companies should double-check their labels, cross-reference regulatory lists, and give new staff a rundown on why that number matters. Government agencies could encourage or require CAS numbers on safety paperwork, not just ingredient lists. When everything lines up, work moves smoother, and safety steps don’t get skipped.
Every field has its headaches, but trying to sort out a chemical mix-up ranks high on the list. No one wants guesswork about what they’re handling. Learning to use CAS numbers avoids those headaches. In the case of 2-Butylamine, knowing its registry number 109-73-9 can mean the difference between a straightforward day in the lab and something much less manageable.
2-Butylamine pops up in a lot of chemical supply catalogs. It’s an organic compound, a clear liquid with a sharp smell you want to avoid. Companies turn to it for making dyes, drugs, rubber chemicals, and even some pesticides. Anyone who’s cracked a chemistry textbook has seen compounds like it. Most people, though, never bump into 2-butylamine at home or on the street.
Yes, it’s hazardous, especially if you get careless around it. A whiff of 2-butylamine burns the nose and throat. If a little lands on your skin, it doesn’t sit quietly — that spot will start to sting and ache. I once watched a colleague splash a small amount on their sleeve in a lab. That shirt never smelled right afterward, and he had to scrub his arm until it looked like he’d tangled with sandpaper.
Eyes complain quickly, too. If it splashes up, you need an eyewash station and quick reaction. Nobody should treat this chemical like vinegar or a household cleaner. 2-Butylamine’s safety sheet labels it corrosive and easily flammable. Vapors build up in closed spaces, setting the stage for nasty accidents unless there’s real airflow.
In industry stories, workers remember headaches and dizziness from breathing even low levels of butylamine. Those are your body’s signals: get out or get protection. Some studies have shown high exposure can harm the liver, kidneys, and nervous system in lab animals. It makes sense. The body isn’t built to keep up with repeated chemical stress.
Accidents happen anywhere, and those moments stick with you. A small leak in a pipe or a careless spill turns a smooth workday into hours of cleanup and paperwork before the mess gets fixed. The stress after you realize what could have happened feels heavy. A chemical like this won’t care if you were just distracted for a second.
Nobody can ignore chemicals like 2-butylamine. They’re part of making things we all use. That doesn’t mean we shrug off the hazards. I’ve seen major plants run tight training programs, pushing every worker to use goggles, gloves, and real ventilation. Schools teach safe lab habits from day one, and for good reason. But outside the professional world, a container left unmarked or unsealed can wind up where it shouldn’t, turning a routine cleanup into a visit to the ER.
Regulators in most countries sort 2-butylamine into categories like “corrosive,” “flammable,” and “toxic.” Companies have to keep storage records, follow disposal rules, and hold emergency response plans. But policies don’t mean much until they meet real-world habits. Even the slickest safety manual fails if nobody reads it.
Training and the right gear help, sure, but real safety grows from the ground up. Quick supervisors spot a loose cap or a careless step and call it out — not to play gotcha, but to remind everyone why they want to go home with all their senses and skin intact. Speaking up matters more than silence.
If you see a label marked “2-butylamine,” the right move isn’t to poke, sniff, or see what it does. Call someone who knows chemicals. In the hands of professionals using careful procedures, 2-butylamine does its job. Anywhere else, it’s just trouble waiting to happen.
Keeping chemicals like 2-Butylamine around brings more challenges than some folks expect. It’s a clear liquid with a sharp, fishy smell, and that odor already hints at how tough it gets if the storage isn’t on point. 2-Butylamine has a low flash point, which just means even a little heat can get it to ignite. It also reacts with common things like acids, oxidizers, and even the air’s carbon dioxide, causing pressure to build in sealed containers. These aren’t tiny risks—they’re the sort of issues that have led to serious accidents in the past.
I spent some years in a lab where 2-Butylamine was on the shelf. Countless times, I pulled out a drum only to find the cap tighter than expected, almost bulging. Turns out, if a container isn’t completely airtight—or if air sneaks in anywhere—pressure builds and a regular cap just won’t hold up. Venting these containers straight into a fume hood kept everyone safer. Once, a neighboring facility skipped that detail and ended up with a busted bottle, ammonia stench everywhere, and a scramble to evacuate. Sometimes avoiding disaster is just respecting the stuff, not cutting corners.
The chemical needs a cool, dry, dedicated spot, built with ventilation in mind. If 2-Butylamine sits in a warm warehouse or a cramped closet, you’re just inviting problems. I’ve seen what happens if storage gets too hot—the vapor builds, and it doesn’t take much friction or static to trigger a fire.
Keeping it in a flammables cabinet, grounded for static, stops sparks before they start. I learned pretty fast that metal shelves weren’t great; a minor acid spill a shelf up could eat through and trigger releases. Cabinets made for storing organic bases stand up better and come with spill trays, so any leaks don’t spread all over.
Mislabeling is a rookie mistake, but it’s more common than you’d hope. I remember a trip to a plant in Texas, where a temporary worker topped up a generic “amine” jug, not realizing it was 2-Butylamine. The result: a nasty reaction when the wrong neutralizer was added, because no one took proper notes. Keeping containers properly labeled isn’t just bureaucracy; it saves real lives.
Water turns 2-Butylamine from risky to out-of-control. If someone stores it somewhere humid, or if fire sprinklers douse a shelf, you can get fumes or an exothermic reaction in seconds. Water-reactive chemicals like this belong far from emergency equipment that uses water. I’ve set up storage where spill kits, fire blankets, and even buckets of dry sand are nearby, but water is kept well clear.
You can create shelves with chemical-proof paint, set ventilation, and label drums, but if nobody in the building knows why those things matter, it’s pointless. Regular checks matter as much as any fancy cabinet. Assigning specific people to log inspections every week keeps on top of leaks or pressure build-up. Sometimes it just means scanning the shelf for clouded liquids or swollen drums.
2-Butylamine isn’t a mystery if you treat it with respect. Good storage saves headaches, prevents harm, and keeps business moving without avoidable emergencies. It’s not about fear—it’s about building habits that make dangerous chemicals a little less frightening.
Looking at any chemical name, you get tossed into a world full of numbers, prefixes, and endings that seem designed to confuse everyone not wearing a lab coat. Usually, a name like “2-Butylamine” tells you a fair amount about the molecule’s structure, once you’ve built up the courage to break it down.
I remember sitting through my first organic chemistry lecture, surrounded by the scent of markers and the nervous energy of twenty people trying not to look confused when a professor asked, “What does 2-butylamine look like?” Most folks, including me, hesitated. “Butyl” means a four-carbon chain, and “amine” means there’s at least one nitrogen atom bonded in there somewhere. The “2” clues you in that the amine group isn’t just thrown onto the end; instead it’s linked to the second carbon.
If you forget how the formula gets put together and just try to memorize answers, you end up missing what chemistry is actually about. Out in the real world—pharmaceutical labs, environmental testing, food safety—it’s not just about getting the formula right on some test. It’s about making sure compounds can get tracked accurately, tested for safety, or flagged if they’re dangerous. Sometimes, the difference between two formulas shifts a medication from useful to toxic.
For butylamine, you’re staring at a molecule that’s used for dyes, pharmaceuticals, and even certain pesticides. Its hazards include skin and respiratory irritation, so knowing what it is—and how to identify it—matters for lab hands and folks downwind from chemical plants alike.
You get to the formula step by step. Start with the four carbon backbone (butane—C4H10). Swap one of those hydrogens for an “-NH2” group, creating C4H11N. The point is, if you can count how to build up a molecule, and visualize swapping atoms around like building blocks, you can spot patterns in all sorts of substances. It’s a simple trick I kept coming back to as chemistry labs got more complicated: Name, count, swap.
You might get lost in countless formulas at first, but following this process opens up the logic behind why the formula for 2-butylamine comes out as C4H11N.
Many students and younger scientists get stuck on roadblocks like chemical nomenclature, memorizing rather than understanding. Then, when work gets handed off in the form of production records or safety data sheets, misunderstandings snowball. If more teachers slowed down and encouraged stepwise breakdowns, more students would actually see chemistry as a practical tool, not a wall of jargon. It also helps to spend a little time on molecular models—real, tangible objects that you can twist and snap together to see changes in three dimensions.
For anyone out there still learning, don’t get discouraged by the technical fog in chemical names. Armed with a little patience and a willingness to count carbons, hydrogens, and nitrogens, you’ll find yourself much more confident going into labs or tackling science reports. That simple formula, C4H11N, holds more value than it looks—the key is getting comfortable piecing it together, one atom at a time.
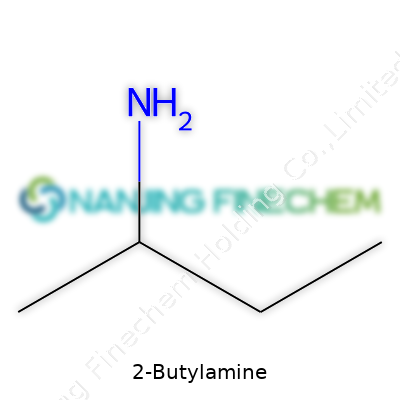
| Names | |
| Preferred IUPAC name | butan-2-ylamine |
| Other names |
1-Aminobutane 1-Butanamine Butylamine n-Butanamine n-Butylamine |
| Pronunciation | /tuː ˈbjuːtɪl.əˌmiːn/ |
| Identifiers | |
| CAS Number | 109-73-9 |
| Beilstein Reference | 1718730 |
| ChEBI | CHEBI:42593 |
| ChEMBL | CHEMBL15739 |
| ChemSpider | 12719 |
| DrugBank | DB14143 |
| ECHA InfoCard | ECHA InfoCard: 100.003.911 |
| EC Number | 206-455-4 |
| Gmelin Reference | **7,107** |
| KEGG | C01858 |
| MeSH | D001072 |
| PubChem CID | 8035 |
| RTECS number | EB2975000 |
| UNII | K4J5G9ZL0C |
| UN number | UN1125 |
| Properties | |
| Chemical formula | C4H11N |
| Molar mass | 73.15 g/mol |
| Appearance | Colorless liquid |
| Odor | ammonia-like |
| Density | 0.742 g/cm³ |
| Solubility in water | miscible |
| log P | 0.89 |
| Vapor pressure | 2.8 kPa (at 20 °C) |
| Acidity (pKa) | 10.7 |
| Basicity (pKb) | 3.29 |
| Magnetic susceptibility (χ) | -6.1 × 10⁻⁹ cm³/mol |
| Refractive index (nD) | 1.399 |
| Viscosity | 0.411 cP (20°C) |
| Dipole moment | 1.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 217.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -53.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3967.6 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02,GHS05,GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H225, H302, H312, H314, H332 |
| Precautionary statements | P210, P261, P264, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 3-3-2-Ac |
| Flash point | 23 °C |
| Autoignition temperature | 310 °C |
| Explosive limits | 1.7% - 10.3% |
| Lethal dose or concentration | LD50 oral rat 367 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 367 mg/kg |
| NIOSH | KJ8225000 |
| PEL (Permissible) | PEL: 5 ppm (parts per million) |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | 300 ppm |