Chemists first described 2,6-Dichlorobenzonitrile in the backdrop of great change in the chemical industry. Early in the 20th century, excitement surrounded new benzonitrile derivatives. Researchers figured out processes that swapped hydrogen atoms for chlorine on aromatic rings, driven by the desire to find potent weed killers and versatile intermediates for more complex chemistry. I remember sifting through old chemical engineering journals, seeing how these compounds shifted from being minor academic interests to key players in agriculture and fine chemicals. As patents emerged in the 1960s, especially those connected to the herbicide dichlobenil, 2,6-Dichlorobenzonitrile grew in commercial significance, carving out its position both in labs and on fields.
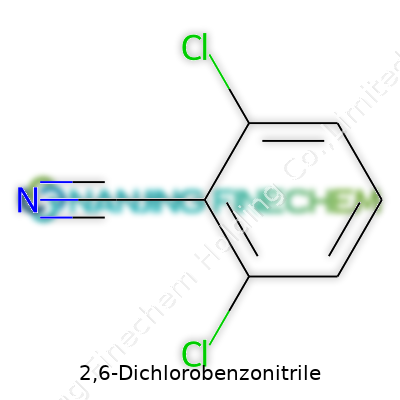
Today, 2,6-Dichlorobenzonitrile handles a wide range of roles. As a white crystalline powder, its status as an intermediate and active agent drives usage in herbicide formulas, especially products aimed at stubborn perennial weeds. The structure—a benzene ring, two chlorine atoms at the 2 and 6 positions, and a nitrile group—gives the molecule its punch, whether in synthesis or action against unwanted plants. This compound finds its way into the production of specialty chemicals, and sometimes even into research settings where new organic reactions get tested for efficiency, yield, and purity. In my own college research, seeing it on the reagent shelf meant whatever we made after would stand up to the rigors and surprises of further derivatization.
With a melting point near 141°C, 2,6-Dichlorobenzonitrile stays solid under most storage conditions. This also means its handling remains manageable, making accidental spills less likely to vaporize. Solubility in water stays low, due to the compound’s aromatic and chlorinated structure, while organic solvents like acetone or toluene dissolve it easily. Chemically, the two chlorine atoms greatly influence the compound's reactivity, deactivating the aromatic ring and steering most reactions toward nucleophilic rather than electrophilic attack. I’ve found the compound to be stable in sealed containers for years—a testament to its durability under most storage and usage scenarios.
Industrial and laboratory suppliers focus on purity, often supplying 2,6-Dichlorobenzonitrile at or above 98% assay. Impurity profiles usually mention trace chlorinated benzonitriles, with water content kept below 0.5%. Packaging info states CAS Number 1194-65-6, molecular weight 172.02 g/mol, and UN numbers where applicable for hazardous goods. Sensible labeling practices warn about inhalation and skin contact risks, referencing its R/S phrases and pictograms mandated under international GHS guidelines. These technical details become crucial for anyone responsible for chemical inventory, not just those running procedures in the lab.
Two main approaches have stuck: direct chlorination of benzonitrile and Sandmeyer-type reactions starting from 2,6-dichloroaniline. Both paths demand close control of conditions—too much chlorine and you’ll over-chlorinate, too little and yields drop. Alkali catalysis sometimes shortens reaction times, but heat control remains tricky. The extraction usually takes place using organic solvents followed by distillation or recrystallization. I remember wrestling with glassware during scale-up, since product crystallization can clog filters if temperature drops too fast. Some plants use closed systems, cutting operator exposure and reducing environmental release.
2,6-Dichlorobenzonitrile serves as a launching pad for many downstream products. Nucleophilic substitution of chlorine atoms, often by amines or alkoxides, gives rise to substituted aromatic compounds with new pharmacological or agricultural action. Hydrolysis leads to 2,6-dichlorobenzoic acid, another versatile intermediate. I’ve worked with students exploring reduction of the nitrile group, making the corresponding amines. Each modification builds on the core stability of the molecule, yet opens the door to new reactivity depending on reaction conditions—temperature, solvent, and nucleophile all make big differences in the outcome.
Industry circles refer to 2,6-Dichlorobenzonitrile by names like Dichlobenil and Caswell No. 301D. Trade names appear in herbicide markets, with products often sold under alternative names depending on regional distributors. Common synonyms like Benzonitrile, 2,6-dichloro-, or 2,6-Dichlorobenzenecarbonitrile show up in literature, underscoring the importance of double-checking structures before placing orders or running analyses. I’ve seen confusion arise in regulatory paperwork and border shipments all because someone missed a synonym.
Working with 2,6-Dichlorobenzonitrile doesn’t call for heroics, but it demands respect. The compound irritates the skin, eyes, and respiratory tract. Chemical-protective gloves, safety glasses, and fume hoods remain standard in my experience, especially when weighing or grinding the solid. Handling protocols focus on minimizing dust, since inhalation presents the most immediate risk. Storage rules call for cool, ventilated areas, far from acids and bases that might spark unwanted reactions. Spill response plans go beyond basic mop-ups; specialized absorbents, respiratory protection, and careful waste containment keep accidents from escalating.
The main spotlight falls on agriculture, where 2,6-Dichlorobenzonitrile acts as a pre-emergence herbicide. It suppresses annual and perennial weeds, with performance favoring non-crop areas, orchards, and berry fields. Landscape professionals appreciate its effectiveness in rail beds and public parks where manual weeding falls short. Beyond weed control, the compound joins the toolbox of organic chemists transforming it into dyes, pharmaceuticals, and other agrochemicals. My mentor shared stories of factories scaling up production in Eastern Europe for both direct herbicide use and as an intermediate for new drug candidates.
R&D teams continue to comb through 2,6-Dichlorobenzonitrile’s properties and applications, searching for safer, smarter derivatives. Work in catalysis looks at greener synthesis using less toxic metals and milder conditions. Analytical chemists probe residues in soil and water, optimizing detection for regulatory purposes. I’ve read studies tweaking the molecular structure, hoping to improve selectivity and lower environmental persistence. Patents still flow for new uses, especially where resistance management in agriculture pushes scientists to remix the classic herbicide toolkit.
Animal studies show moderate acute toxicity, with neurological effects at higher exposure levels. Chronic exposure risks center on potential for liver and kidney stress, but target organ toxicity stays less pronounced compared to older, more notorious herbicides. Environmental researchers watch aquatic impacts, since the molecule resists rapid breakdown in water and can drift from treated soil. Field reports point to occasional non-target plant damage, driving calls for tighter application rates and buffer zones near wetlands or sensitive crops. I’ve witnessed regulatory shifts in some countries after water quality testing linked traces in runoff to the use of dichlobenil-based formulations.
2,6-Dichlorobenzonitrile won’t fade from view soon. Pressure grows for sustainable weed management, and synthetic chemicals still fill gaps that hand-weeding, mulching, and biocontrol leave behind. Biochemists search for ways to tweak the core structure for eco-friendlier breakdown or reduced mobility in soil. Demand grows for specialty intermediates as pharmaceutical research zeroes in on halogenated aromatics. Learning from mistakes of the past, newer formulations target reduced drift, higher application precision, and lower persistence in the environment. Personally, I expect to see this molecule spark new debates—and innovations—across agriculture and chemistry classrooms alike.
Walk into any supply shop for farmers, and you’ll likely spot a handful of products containing 2,6-Dichlorobenzonitrile. Few outside the industry recognize the name, yet this chemical has become a staple in the toolkit for weed control. Ask long-time growers, and many recall the first time they watched it knock down stubborn grasses that otherwise outcompete seeds and leach away nutrients.
This compound found its place over the years because it hits weeds before they hit crops. 2,6-Dichlorobenzonitrile gets into the soil and stops weed seeds from sprouting. Physical weeding only goes so far, especially for those with hundreds of acres or community parks to manage. I’ve seen neighbors struggle for years yanking out crabgrass and pigweed until a soil treatment finally turned the tide.
Products using this chemical cut down on labor. No one wants to spend days pulling up growth by hand. A single treatment across a field often saves hours of work later. Less sweat on weeding means more time for tending fruit or prepping for market.
Harvest depends on how well a crop competes for water and sunlight. Unchecked, weeds choke out the good plants and can ruin a whole season’s effort. 2,6-Dichlorobenzonitrile gives tomatoes, beans, and cucumbers a fighting chance by knocking out the competition before it can get going. One summer I tried out a treated patch next to an untreated patch; the difference still sits fresh in my mind. The rows protected with this molecule delivered plumper tomatoes and cleaner pathways.
Landscapers also turned to this tool for aesthetic reasons. It keeps decorative beds tidy at office parks, churches, and sports fields. Mulch suppresses some invasive plants but not all. Adding a pre-emergent like this makes those high-traffic spots easier to maintain and cheaper to keep looking sharp.
No one likes to talk only about the upside of chemicals. Stories pop up about residues or the risk to nearby streams during runoff. My uncle, a conservationist, reminds me that careless use can harm fish and pollinators. That means anyone using 2,6-Dichlorobenzonitrile faces a responsibility. Reckless handling risks fines and even bans in some regions.
Crop rotation and buffer zones can help. Some forward-looking growers test alternative methods alongside chemical options. Mechanical weed barriers, hand-weeding in sensitive plots, and careful timing reduce the load. Workshops hosted by university extension offices now address safe handling, as well as options for reducing chemical use without going back to endless digging.
Regulations push users toward tighter controls. Labels call for protective gear and strict timing to avoid runoff during rain. That may raise costs, but it protects both yield and soil health over time. Each year brings updated best practices, and groups from local ag cooperatives to state agencies share results from new trials and experiments.
Weed management looks different now than it did even a decade ago, thanks to chemicals like 2,6-Dichlorobenzonitrile. The real winners are those growers willing to blend good chemistry with new tricks, always looking for better ways to balance crop success and land stewardship.
2,6-Dichlorobenzonitrile keeps things simple: its molecular formula is C7H3Cl2N and the unique CAS number connecting researchers and manufacturers to the right chemical is 1194-65-6. Those numbers aren’t just trivia—they guard against mix-ups in research and industry alike. No two substances share a CAS number, so anyone pulling a request or placing an order avoids nasty surprises in their bottles.
Small facts like a molecular formula and CAS number don't always get a spotlight, but they cut straight to the point behind a lot of what’s safe and accurate in chemistry. There’s a reason you won’t see folks stumbling into labs with a recipe written out by name only: everything gets double-checked, usually with that very CAS number. I remember a younger chemist in the lab, keen to impress, ordering what he thought was a related compound by skimming the product name. The mix-up wasn’t dangerous but delayed a project by weeks and racked up some waste most environmental officers would scold. That little slip taught everyone to double back and confirm molecular ID, not just take a label at face value.
2,6-Dichlorobenzonitrile doesn't pop up in daily conversation, but you might spot it working out in the real world. It sits in herbicides, keeping unwanted plants in check. Farmers trust the right formulation for specific crops, relying on tight regulations and safety checks. If the molecular details drift, wrong batches could ruin a season’s yield or risk an ecosystem shakeup. This isn’t some distant lab concern either; the stories I hear from growers always mention how one bad chemical delivery triggers sleepless nights. Exact numbers become a backbone for predictability in their fields—there’s no room for margin-of-error mistakes.
Mixing up chemicals with similar names or swapping a digit in a CAS number doesn’t just confuse supply chains. Subtle differences change how substances react with others, how they break down, or how much danger they bring. In regulatory paperwork, these tiny data points form the backbone of safety sheets that protect workers, transporters, and folks nearby. Misidentifying a chemical creates more than a paperwork mess—it sends risk cascading, sometimes right into homes and schools downstream from a plant or field.
In my experience, every chemist, farmer, and technician benefits from a healthy obsession with details. I keep a laminated card of common target molecules and their CAS numbers at my desk, not out of paranoia but because it pays off in speed and certainty. Double-checking these identifiers—kind of a second nature, like looking both ways before crossing a street—saves from careless mix-ups. Digital systems can flag discrepancies in orders, but human eyes often catch what a computer misses, especially if a supplier adds or drops a letter. Education and attention to these basics keep unnecessary accidents off the news and out of the emergency logbook.
Step into a pesticide or chemical warehouse and you'll hear plenty of talk about 2,6-Dichlorobenzonitrile. Not every worker knows its story, but a few seasoned hands always watch out for its drum in the back. I've seen how easy it is to underestimate this pale crystal with a bland name. No one brags about storing it since real danger often comes from those bottles no one remembers.
Ask around, and a few folks can tell you that this compound means business. Used in herbicides, it's not only rough on weeds—it's unfriendly to skin, eyes, and lungs. You might hear managers call it "moderate" on the risk scale, but I’ve seen the reaction when someone slaps a contaminated glove on their wrist or takes a whiff by accident. Red eyes, sore throats, and worse—we don’t shrug those off. I've read clinical notes tying this stuff to nasty coughing fits and headaches, not to mention the environmental mess if it leaks out.
This isn't just a checklist chore. I’ve worked in places that tossed chemical safety sheets in the drawer, and I’ve worked where people care. There’s a big difference. Keep 2,6-Dichlorobenzonitrile containers sealed up tight. Humid air can get inside. Moisture clings, causing lumps in the product and even corrosion on metal lids. Dampness also invites minor chemical breakdown—introducing byproducts you don’t want to mix with the next batch.
Never line this up with food, feed, or open drink containers. It doesn't smell strong, but that means it sneaks up on folks. I always say: separate rooms, clear labeling, and sturdy shelves. Minimize direct sunlight hitting the bottles. UV can chew up chemical stability and produce odd odors—sometimes those are your only early warning. I’ve seen labels fade to blank after just a few months near a warehouse window. That’s a bad day for anyone looking for lot numbers in a recall.
A stuffy closet doesn’t cut it. 2,6-Dichlorobenzonitrile gives off dust and can go airborne during careless handling. I always crack a window or lean on a fan, especially during summer. Personal protective equipment isn’t a hassle; it’s as natural as grabbing your car keys. Nitrile gloves, long sleeves, splash goggles—these are basics. If you’ve ever felt the sting from an accidental splash, you never forget again. I keep a few extra respirators on hand. Sometimes newbies want to tough it out. It’s easier to hand one over than argue about safety.
No matter how careful you are, a jar can slip. It’s the panic afterward that decides whether things get worse. I’ve trained with spill kits—absorbent pads, chemical neutralizers, non-sparking tools. Paper towels are no substitute. Sweep up crystals with care and avoid tossing contaminated gear into everyday trash. If something goes wrong, folks need to know who to call and where to bring first aid. Fast action stops major headaches.
You can tell which teams have got it figured out. They walk into storage rooms with respect, not fear. They train the new hires like they're family. Regular safety reviews and honest talks about near-misses make more difference than any poster on the wall. If more people take these safety steps seriously—storage, labeling, gear-up, and cleanup—there will be fewer accidents and a better day for everyone on the floor.
Ask anyone who’s worked in manufacturing or agriculture—chemical names aren’t just lines on labels. 2,6-Dichlorobenzonitrile, better known in some circles as dichlobenil, gets used as a herbicide. Walk through garden supply stores or old tool sheds and you’d run into weed control products loaded with it. Nobody strings up warnings for nothing, so a closer look matters.
Nitriles don’t belong around kids or pets. I remember reading a study where skin contact left folks with redness, sometimes even burns if exposure lasted too long. Breathing it in wasn’t any better: headaches, sore throat, coughs—the usual suspects when chemicals float in the air. Past health records from workers show patterns: accidental splashes have led to trips to doctors. Science backs this up. The National Institute for Occupational Safety and Health (NIOSH) tags this compound as hazardous. Nobody wants to risk chronic lung or skin issues just to clear weeds.
Pouring garden chemicals straight onto the ground leaves a mark. Dichlobenil hangs around in soil for months. Farmers and researchers checked water quality downstream and flagged contamination—traces of this chemical end up in rivers. Fish don’t take kindly to it. European environmental agencies even list it as toxic for aquatic life. Dead zones along local creeks aren’t just fiction; they’re small disasters in the real world.
Weed control brings in returns on crops, but the trade-off can hit home years later. One year, fields get a boost and weeds stay down. But soil tests years later reveal the compound remains, breaking down far slower than expected. Trying to rotate crops or bring fields back for vegetables turns up these leftovers. Home gardeners sometimes see their flowers refuse to thrive in spots where these chemicals linger.
A blanket ban on chemicals never gathers much support, but steps make a difference. Protective gear works wonders. Heavy-duty gloves, masks, changing out of work clothes—old lessons still hold up. It helps if community leaders push for regular soil and water testing near where the chemical gets used. Tracking results over time gives a warning before things hit crisis level.
Switching to physical weed removal methods or organic options isn’t out of reach. Plant barriers and mulching require more sweat, but they don’t poison groundwater or threaten wildlife. Iceland, for instance, shifted away from many persistent chemicals and saw their rivers clear up. No single fix fits everywhere, but small steps from lots of people stack up. Minimizing use, storing leftovers safely, and teaching folks why care is needed protects more than just next season’s yield; it guards the next generation’s water and health.
Every risky chemical asks for a pause. From the field to the dinner table, the marks left by 2,6-Dichlorobenzonitrile thread through more than one season. Relying on short-term gains has a way of piling up problems. Facing the hazards means asking neighbors how they manage, keeping eyes out for warning signs in water or soil, and sharing lessons learned. Responsibility doesn’t mean fear; it means not taking chances with what can’t be undone.
If you’ve ever worked in a lab or spent any time around fine chemicals, 2,6-Dichlorobenzonitrile has a distinct look. Pour it out and it comes as white to off-white crystals or a powder, sometimes giving off a faint smell that's just a little chemical—nothing overwhelming, but it’s noticeable if you get close. Anyone handling it day in and day out knows that any change in color or texture signals something’s off, and that can throw off the entire chain of work downstream.
People tend to picture chemicals as mysterious liquids or aggressive fuming reagents, but here, the simple crystalline nature makes storage and handling less of a hassle. The fact that it clumps or cakes in damp air is a minor inconvenience, not a catastrophe, but it reminds you this is something you don’t just leave open on the bench. Storage away from heat and moisture is a standard practice, usually just a cool, dry shelf with a tightly sealed bottle.
There’s a big difference between “good enough” and “right.” In the chemical industry, especially with compounds like 2,6-Dichlorobenzonitrile, stakes are high. Agrochemical producers, for one, need high-purity materials to produce herbicides that work the way they’re supposed to. The accepted purity floats around 98% or higher – this stems not just from tradition but direct experience. Impurities, even in low amounts, sometimes clog up processes, damage equipment, or end up in the final crop, where nobody wants them.
Labs achieve these levels with repeated crystallization or column chromatography. Documentation for every lot—chromatograms, certificates of analysis—comes from the reality that buyers want proof, not promises. If product falls below spec, it’s set aside or gets cleaned up further. In my time sourcing reagents for a research team, I saw teams skip entire suppliers over a single deviation from expected purity; trust is hard to win back after a poor shipment.
A batch labeled “98% min” means the lowest acceptable purity sits around 98%. The remaining 2% includes trace moisture, byproducts, and leftover solvents. Manufacturers usually specify the melting point, between 140-143°C, as a quick check—samples outside that range indicate impurity. Moisture matters; labs keep it under 0.5%. Certain stubborn contaminants need targeted testing, like GC-MS or HPLC, since nobody wants unknown peaks in their data or unexpected toxins in their products.
Tight control over purity comes from both practical experience and regulatory guidance. Regulators often enforce limits on specific contaminants, especially for any chemical going into agriculture or pharma supply chains. Before broad distribution, companies run stability tests—those yellowed, slumpy powders turning up months later represent risk, wasted money, and possibly rejections at the customer’s door.
Issues pop up in transit and storage. Some suppliers solve the clumping issue by adding desiccants to packaging. Labs fighting cross-contamination run cleaning validation between lots, documenting every step. Analytical teams invest in better instrumentation—a rule born of too many ruined assays due to minor impurities. For the larger supply chain, transparency about impurity profiles—routine audits, open communication—goes further than hiding behind a certificate.
Focusing on hands-on solutions, improving batch reproducibility often comes down to better quality control at the factory. Using modern purification steps and realistic shelf-life dating, suppliers build trust not just with certificates, but with samples that do what’s asked of them, no surprises attached.

| Names | |
| Preferred IUPAC name | 2,6-dichlorobenzenecarbonitrile |
| Other names |
2,6-DCBN Cyanazine INN Dichlobenil 2,6-Dichlorobenzene carbonitrile |
| Pronunciation | /ˈtuː sɪks daɪˌklɔːrəʊˈbɛnzoʊnɪˌtraɪl/ |
| Identifiers | |
| CAS Number | 1194-65-6 |
| 3D model (JSmol) | `JSmol("C1=CC(=C(C(=C1)Cl)C#N)Cl")` |
| Beilstein Reference | 1209955 |
| ChEBI | CHEBI:35633 |
| ChEMBL | CHEMBL20201 |
| ChemSpider | 20033 |
| DrugBank | DB08657 |
| ECHA InfoCard | 100.016.516 |
| EC Number | 204-321-4 |
| Gmelin Reference | 715058 |
| KEGG | C14132 |
| MeSH | D003555 |
| PubChem CID | 8534 |
| RTECS number | GL7875000 |
| UNII | Q6U8F08HAB |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C7H3Cl2N |
| Molar mass | 204.02 g/mol |
| Appearance | White to light beige crystalline powder |
| Odor | Odorless |
| Density | 1.42 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.9 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 16.76 |
| Magnetic susceptibility (χ) | -52.0e-6 cm^3/mol |
| Refractive index (nD) | 1.599 |
| Viscosity | 0.1823 cP (25°C) |
| Dipole moment | 2.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –3.9 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -454.7 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N06AX11 |
| Hazards | |
| Main hazards | Harmful if swallowed, toxic in contact with skin or if inhaled, causes skin irritation, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P333+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2,1,1,0 |
| Flash point | 138 °C |
| Autoignition temperature | 540 °C (1004 °F; 813 K) |
| Explosive limits | Upper: 6.8%, Lower: 1.1% |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1920 mg/kg (oral, rat) |
| NIOSH | SN2100000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 2,6-Dichlorobenzonitrile: Not established |
| REL (Recommended) | REL: 3 mg/m3 |
| IDLH (Immediate danger) | 25 mg/m³ |
| Related compounds | |
| Related compounds |
2,6-Dichlorobenzamide 2,6-Dichlorotoluene 2,6-Dichlorobenzoic acid 2,4-Dichlorobenzonitrile 3,5-Dichlorobenzonitrile 2,6-Dibromobenzonitrile 2-Chlorobenzonitrile 4-Chlorobenzonitrile |